LC8.1. Dissolving Rocks
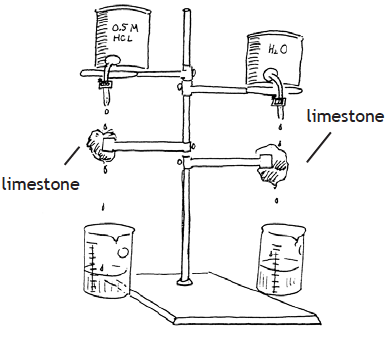
In the laboratory, you can simulate the process of chemical weathering by setting up an arrangement as shown here. This apparatus will allow you to control drops of soda water (carbonic acid, H2CO3) to drip onto limestone (CaCO3) for a few days, and collect the water that has passed over the rock. Keep everything in your experiment the same, except that on one side soda water (carbonic acid) is dripping and on the other plain tap water is used. Next, evaporate the liquids collected by boiling them on a hot plate or Bunsen burner until all the liquid is gone. What is left? (If limestone is unavailable, you can use calcite or marble.)
Extending The Experiment
Work with another lab team to decide on a difference between your two setups that may affect the amount of rock that is removed by dissolving. Differences could be the kinds of rocks used or the frequency, velocity, or size of the water and carbonic acid drops. The purpose of the experiment is to determine if the difference selected has any effect on the results. One setup might use limestone and the other might use marble. Or if both teams use limestone, one team might let the drops fall from a height different from the other.
The two lab setups should be the same except for the one variable. Why?
Reporting Results
After doing the experiment, prepare a lab report that covers the following:
- Describe your experiment. What variable did you test? What was kept constant? How long did the experiment run?
- Did the rocks change? How? Were changes caused by water and a dilute acid the same or different? What did you observe when you evaporated the liquids?
- What did you measure to determine the effect of the experimental variable?
- How did your results compare with another team’s results? What was the difference between your team’s experiment and the other team’s?
Drawing conclusions
- Using the above information and the results of your experiment, explain
- the rounded appearance of weathered rocks;
- the difficulty of reading old tombstones;
- how stalactites (structures of rock-like material hanging like icicles from some cave roofs) and stalagmites (rock-like mounds on some cave floors) are produced.
- What are the implications of your results for how the variable you tested might affect the weathering of rocks, the formation of underground caves, or the global climate? Does comparing your experiment with another team’s give you any more information?
- What part of the carbon cycle is simulated in this experiment? What parts of the carbon cycle are left out?
- Carbon is stored not only on the bottom of the ocean but also in Earth’s crust in the form of coal and oil deposits, and within the bodies of all living forms. How might human activities affect the long-term carbon cycle and what might this effect have on world climates?


