LC3. How Do Scientists Play the Dating Game?

Chapter 3
I. Kinds of Rocks
Earth’s history is written in its rocks. But before the 1780s, no one had figured out how to read that history. In fact, the question of why there were different layers of rock in a variety of formations simply didn’t generate any interest. True, Leonardo da Vinci had speculated that marine shells and fossils found high on a mountain top were the remains of plants and animals that had lived at sea level long, long ago, but how that could be was a mystery.

It wasn’t until the late eighteenth century that people began to understand how rocks formed, which led to the knowledge of Earth’s history that we have today.
One of the first theorists was a German mining engineer and naturalist, Abraham Werner. He believed that in the beginning there was a worldwide ocean which was deep in some parts and shallow elsewhere. Fine particles of sand and mud—sediment—from Earth’s crust compressed as the water subsided, and formed rocks, which came to be known as sedimentary rock. Over time, these rocks accumulated into layers. Werner also believed that the different layers of rocks were formed at different times.
The Scottish naturalist James Hutton, who lived at about the same time as Werner, agreed that some rocks were formed by sedimentation. However, Hutton argued that certain kinds of rocks were formed by the cooling and crystallization of molten rocks that came from deep underground. By the 1820s, geologists (scientists who study rock formations) generally agreed that both Hutton and Werner were right. There are two ways that rocks could form: either they are cooled parts of the molten interior of Earth—the igneous rocks that solidified from lava or volcanic fragments—or they are underwater deposits that have solidified—the sedimentary rocks. Later, scientists recognized that a third kind of rock is created when gradual movement of Earth’s crust reshapes sedimentary and igneous rocks under great heat or pressure. These rocks are called metamorphic.
Sedimentary rocks gave the first clues to the vast age of Earth. The early geologists observed how clay and silt were deposited at the mouths of rivers. If the material deposited is sand, over many years layers of sandstone form. If the material is mostly clay, a finer and harder rock called shale forms. In addition, on the ocean bottom, the tiny shells of sea creatures settle and eventually form limestone.
In places like the Grand Canyon layers of sedimentary rock are thousands of feet thick. Since many types of sedimentary rock form very slowly, a great deal of time must have passed while these rocks formed. When geologists understood how sedimentary rocks were created, they began to realize that Earth must be at least millions of years old. We now know that Earth is billions of years old.
II. Sorting Through the Layers
Geologists determine the sequence of time in Earth’s history by studying sedimentary rock formations. Although all sedimentary rocks were formed at the bottom of a lake or on the ocean floor, today they can be found anywhere, even on mountains! That is because rocks are sometimes pushed up from below.
The arrangement of the strata—the different layers—is called stratigraphy and the principle that is followed in reading the layers is superposition. This means that the layers of rocks are presumed to have been laid down in succession, with younger layers on top of older layers.
The principle of superposition can be illustrated by the following example. If you take off your clothes and throw them in a pile on the floor, someone examining the pile can tell which article of clothing you took off last and which you took off first. If a paleontologist (a scientist who studies the life of past geologic periods) finds a fossil in the top layer of a sedimentary rock section and a different fossil below it, he or she can report that the organism below lived before the one whose fossil was found on top.
While the law of superposition is usually a good indicator of relative age, in some cases the eruption of volcanoes or other earth movements tilt the layers of rock on end, or even flip over huge slabs of sedimentary rock, disturbing the orderly sequence.
In order to draw valid conclusions, geologists generally compare the stratigraphy of different regions to see if they give a consistent picture of what happened when.
The recognition that different layers or strata contain fossils of different living organisms came not from a professional scientist but from a British surveyor named William Smith. He had long been interested in fossils and realized that each layer seemed to have its own kind of fossils. He concluded that a rock layer and the fossils within it were of the same age. That is, the different kinds of plants and animals whose remains fossilized were alive at the same time that the stratum, or layer, was formed at the bottom of a lake or on the ocean floor. The deeper the stratum, the more ancient the plants and animals. Some fossils can be found in many different strata, while others are only in one stratum, corresponding to a certain time period. The fossils that can be used to identify different strata are called index fossils.
For example, English geologists in Cambria, England, identified certain index fossils and called the period of history when those fossils formed the Cambrian period. Much later, similar fossils were found in layers of rock in the Grand Canyon, thousands of miles away. Therefore, this layer of rocks must have formed at the same time as the layer discovered in Cambria, England.
III. How Are Fossils Formed?
The fossils embedded in layers of sedimentary rock formed in various ways. Some are the hard shells and bones of long-dead creatures that were buried between layers of sediment. Soft-tissue fossils formed when leaves, fish, or other living tissues became trapped between layers of clay or other sediment and decayed, leaving a space. Over millions of years these spaces sometimes filled with minerals that leaked through the porous rock and eventually hardened in the shape of the long-dead plants or animals.
More than a century of research on stratigraphy all over the world has led geologists to divide the history of Earth into segments of time. Just as we divide the year into months, weeks, days, and minutes, Earth’s time is divided into eras, periods, epochs, and eons. (See chart below.) These segments of time make up the geologic time scale, which helps us understand geological history in the same way that a historical timeline helps us understand the flow of human history. Each era is divided into periods. These represent important stages in evolution, when new types of fossils were found in the layers of rocks. The two most recent periods, the Quaternary and Tertiary, are further divided into epochs. In later chapters we’ll revisit these periods of time to see how the climate changed and how life evolved.
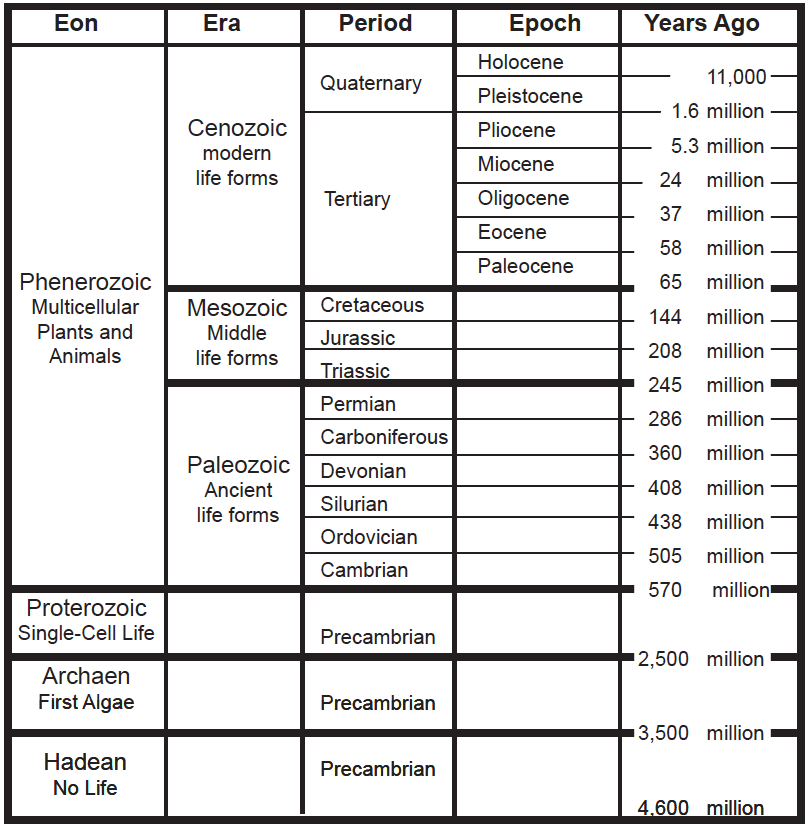
Time periods are distinguished by the kinds of fossils that have been found in certain layers of rocks. Although there are differing interpretations, the broad outlines of the geologic time scale, shown on the next page, are generally agreed upon. We can interpret the readings of the rocks as a work in progress. The rocks and their fossils offer us the best evidence about our planet’s history.
Notice that eon refers to the longest interval of time on the geologic time scale. The entire history of our planet is divided into four eons: the Hadean eon when there was no life at all, the Archaen eon when the first forms of life evolved, the Proterozoic eon when a wide variety of single-celled life evolved, and finally the Phenerozoic eon—the most recent 570 million years, when nearly all of the multicellular life forms existed.
The Phenerozoic eon is divided into three eras. You are probably familiar with the Mesozoic era, when dinosaurs were the dominant life form on our planet. The Mesozoic ended when 50% of all plant and animal families became extinct, including all species of dinosaurs. It is now widely believed that the mass extinction was caused by an asteroid, some ten kilometers in diameter, that struck Earth somewhere near what is today the Gulf of Mexico. An even more catastrophic event occurred at the end of the Paleozoic era, when about 90% of all plant and animal families became extinct. There are many different ideas about what caused that massive extinction—the greatest of all time—but no one really knows for certain what caused it.
Following the Mesozoic era is the Cenozoic era, or the age of mammals—the era in which we live.
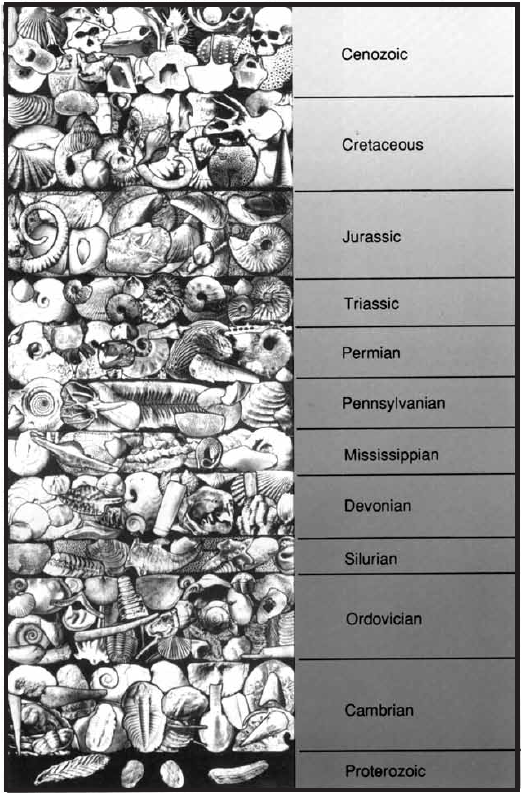
The illustration below is of index fossils is from Fossils, Rocks, and Time, prepared by the U.S. Geological Survey, U.s. Department of the Interior, 1994. Geologists sometimes give geologic periods different names. Compare this chart with the geologic time scale above. What difference do you find in the naming system used in these two charts? Check other textbooks and reference books to see if you can find other differences in the names given to geologic periods.
V. Early Dating Methods
Once the clues in the rock strata were understood, scientists were able to describe a fairly detailed outline of Earth’s history. The various geologic periods were known from the rock strata, but just how long ago each layer formed remained a mystery.
In the nineteenth century one method of estimating the passage of time was to observe the formation of sediments at the mouths of rivers and on the ocean floor, and to measure how rapidly the sediment was deposited. These observations made it possible to infer how long it took for the layers of rock to form. This method yielded age estimates in the millions of years, but there were too many variables. For example, in some areas the rivers flowed much more rapidly than in other areas, so layers of sediment formed more quickly. Weather also made a difference. During floods the rivers carried much more sediment than during droughts, when there might be no sediment formation at all. These time estimates were therefore unsatisfactory. It wasn’t until the twentieth century that an accurate time scale could be determined. But the story began a few years before, with the discovery of X-rays.
VI. The Discovery of X-Rays
Science is a process that builds on what is known (or thought to be known) to explore the unknown. In 1896, Wilhelm Roentgen, a German physicist, announced his discovery of a previously unknown ray that could pass through opaque substances, such as paper, wood, and even human flesh. He named the phenomenon the X-ray because he did not know its source. Roentgen’s discovery was sensational and received worldwide attention.
Following the discovery of X-rays, the eminent French physicist Antoine Henri Becquerel began a series of experiments to discover the source of X-rays, and in the process he discovered something else. He started by using uranium salts, a compound containing the element uranium which is phosphorescent—it glows in the dark after being exposed to sunlight. In his first few experiments he wrapped a photographic plate with black paper so that no light could penetrate to darken the film. He laid uranium salts that had been exposed to the Sun on top of the black paper. When he developed the film, he found a dark spot where the uranium salts had been. The dark spot told him that rays from the glowing uranium salts must have penetrated the black paper.
Photo courtesy of the Health Physics Society
When Becquerel attempted to repeat the experiment, he again wrapped a photographic plate in black paper. He put a thin piece of copper in the shape of a cross on top of the black paper and sprinkled the top with uranium salts. He expected that when the uranium salts were exposed to sunlight they would absorb energy from the Sun, and then give off X-rays. The X-rays would darken the photographic plate, except where the copper cross was located.
Unfortunately, it was a cloudy day, so he placed the material in a cupboard for later use. The weather continued to be poor for several days. Out of curiosity he developed what he thought was a useless plate and found that it had darkened anyway, except where it was covered by the copper cross. Becquerel reasoned that something in the uranium salts must be producing rays that could penetrate the black paper and darken the plate, even without sunlight to make the uranium salts glow. That meant the rays—whatever they were—came from something inside the uranium itself. The rays could penetrate paper and darken film, but they could not penetrate a thickness of copper.
Becquerel reported to the French Academy of Sciences that he had found “rays of a peculiar character” coming from uranium compounds. He continued experimenting and wrote more papers about his findings, but his work received little notice. It seemed as if everyone’s attention was focused on Roentgen’s X-ray. Partly because Becquerel’s mysterious rays were neglected and offered something new to investigate, Marie Curie decided to make them the subject of her doctoral dissertation.
VII. The Curies’Discovery of Radioactive Elements
Marie Curie was an extraordinary person. She grew up in Poland at a time when women were not allowed to attend college. She and her sister joined a group of young people in Warsaw who met secretly in homes of supporters to discuss new ideas in politics, philosophy, and science. Eventually, in 1891, she moved to Paris where she enrolled at the Sorbonne, which was one of very few universities to admit women. After three years of constant study, she took the exams for a Master’s degree in physics, and was first in her class. The next year she earned another Master’s degree in mathematics. In 1895 she met and married a young French physicist, Pierre Curie, who had already made important discoveries of his own.
As her lab notebook showed, Marie Curie started her research on Becquerel’s mysterious uranium rays in December 1897, and in February 1898 she tested various compounds, including pitchblende, a rock that contains the element uranium. Surprisingly, the pitchblende produced even more of the rays than pure uranium. She reasoned that there must be an element in the pitchblende that produced even more of the rays than uranium. That is, it was more radioactive, a word invented by the Curies.
At this point Madame Curie’s husband, Pierre, joined in the research. Together, their focus was on investigating pitchblende to find out what the radioactive substance was. In order to do that, they had to separate the radioactive substance chemically from the rest of the pitchblende ore. This was done by a method that Madame Curie invented called fractional crystallization. The process involved grinding and dissolving the minerals in the pitchblende, then heating and cooling the liquid until crystals formed—similar to the way rock candy is made by crystallizing sugar. Since crystals of different minerals form at different temperatures, she was able to separate different substances and test each of them for radioactivity. In this way the Curies identified two different radioactive substances: polonium, which they named for her native Poland, and radium, which was strongly radioactive.
The Curies were not members of the influential Institut de France, the academy of science where such important findings would be reported. However, Becquerel recognized the importance of their discoveries and presented their report for them. Later, other scientists presented papers for the Curies.
The Curies continued their hard work for three more years under difficult conditions. Originally, their laboratory was in the unheated storage area of the school in which Pierre Curie taught. Then, they moved to a larger but poorly equipped space with a leaky roof. Despite the limitations, they were able to carry out the careful and tedious work of reducing several tons of rock to tiny samples of pure polonium and radium.
Madame Curie completed her dissertation with a description of their work and in 1903 was awarded a Doctor of Philosophy degree from the Sorbonne. Soon after, the Curies shared the Nobel Prize in physics with Becquerel. Their work was going well, but unfortunately, just three years later Pierre was killed. He had collapsed in the street and fell under the wheels of a horse-drawn vehicle that was unable to stop in time. Whether his exposure to radium contributed to his collapse is unknown, since no one knew how dangerous radioactivity is. Madame Curie took over his teaching duties at the university and became the first woman professor of chemistry. She continued her work with radioactive elements, and in 1911 she was awarded her own Nobel Prize in chemistry. She died in 1934 of leukemia.
It is interesting to note that in 1897 Madame Curie gave birth to her daughter Irene. After Irene grew up, she became an assistant in her mother’s laboratory and married another research worker there, Frederic Joliot. He added the name of Curie to his own because his in-laws had no sons to carry on the Curie name.
Working together, Irene and Frederic Joliot-Curie discovered how to make elements that were not radioactive produce radioactive rays. They received the Nobel Prize in chemistry in 1935. Irene died in 1956 of leukemia, the same disease that had killed her mother. It is believed that their leukemia might have been caused by overexposure to radioactive rays.
What Radioactivity Meant to Geologists
The discovery of radioactivity made it possible to finally measure the age of rocks and then to construct a time scale of Earth’s history. Although rock types had been recognized for some time and fossils helped in identifying which strata were older and which were younger, determining the age of Earth using a scientific method was not possible before the discovery of radioactivity.
VIII. Radioactive Decay
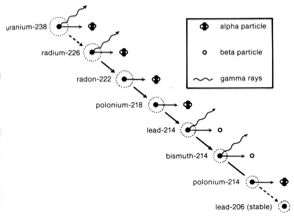
There are 92 naturally occurring elements. Most of these are stable. That is, the atoms of these elements retain their constituent parts—protons, neutrons, and electrons—indefinitely. However, radium, uranium, and a few other elements spontaneously eject particles from the interior of the atom and become a different kind of element. An atom of uranium, for example, gives off several particles and becomes an atom of lead. This process is called radioactive decay.
The atoms in a radioactive element do not decay all at once. It is impossible to predict when any single atom will decay. However, the rate of decay—the speed at which a sample of uranium turns into lead—can be measured and predicted. A good analogy is the prediction of automobile accidents. It is impossible to predict when any given automobile will be involved in an accident. However, it is not difficult to predict approximately how many accidents will occur, on average, every day in the United States.
Rate of decay for radioactive materials is generally given in terms of half-life. Half-life is the amount of time it takes for half of the atoms in a sample to decay. The half-life of uranium is 4.5 billion years. Measurements of the relative amounts of uranium and lead in very old rocks and meteorites have led to the conclusion that Earth formed about 4.6 billion years ago.
Another element of great use to geologists is a radioactive form of potassium that gradually turns into argon—a gas. The half-life of radioactive potassium is 1.3 billion years. Potassium is one of the constituents of the mineral mica. Mica is sometimes found in layered sheets as a brittle, transparent mineral. Like other crystals, mica formed out of molten rock. While it was molten, bubbles of argon gas would have escaped. But after the mica crystallized, the argon would have been trapped inside. So, the method of potassium-argon dating establishes how long it has been since the material being analyzed was molten.
IX. Conclusion
Both stratigraphy and radioactivity are essential tools in figuring out the geologic time scale. Only some isotopes of some elements are radioactive. Of those, only a few have a half-life that is long enough to be useful in dating. Therefore, geologists are not able to find accurate dates for all rocks. However, by dating rock layers that do contain pitchblende, mica, or other rocks that contain radioactive isotopes, geologists can then infer the ages of the rock layers in between.
Thanks to the discovery of radioactivity by Antoine Henri Becquerel, Marie and Pierre Curie, and many others, geologists can now determine the age of certain types of rocks and gases. Wherever such rocks or gas bubbles are found embedded in a rock formation, the age of the formation can be determined. This approach has made it possible to construct the geologic time scale.

LC3.1. Investigation:
Simulating Half-Life Decay with Pennies
In order to better understand how the idea of half-life is used in dating rocks, you can experiment with a simulated radioactive substance that we will call “pennyonium.” A sample of pennyonium consists of 100 pennies. Atoms of pennyonium decay to lead by emitting high energy particles. In this activity you will need to work as a member of a 2- or 3-person team.
Materials
100 pennies
1 small box with a lid
Procedure
- Create a chart and graph for collecting data as shown below.
| Number of Years Since Sample Formed | Atoms of Pennyonium Remaining |
| 0 | 100 |
| 1,000 | |
| 2,000 | |
| 3,000 | |
| 4,000 | |
| 5,000 | |
| etc. |
- Put all the pennies into the container. Imagine that the pennies represent atoms of pennyonium, which has a half-life of 1,000 years.
- Put on the lid, shake the container, and drop the coins onto a table. Remove the coins that are tails up. These coins represent the atoms that have decayed to lead during the first 1,000 years.
- Count the number of heads-up coins remaining. Enter this number in the data table to show how many atoms of pennyonium remain.
- Repeat steps 2 and 3 until there is just one coin or none remaining. Each shaking represents another 1,000 years.
- Graph your results. On the vertical axis plot the number of coins remaining after each period of 1,000 years. On the horizontal axis plot the number of years that took place in your experiment.
Questions For Thought
- Compare your graph with graphs of other teams. Try to explain similarities and differences that you notice.
- How many years did it take for the sample to decay from pure pennyonium to lead?
- How many years passed before approximately half the sample decayed?
- Imagine that you find a sample of pennyonium in a layer of rock and analyze it. You find that out of 100 atoms, 13 are pennyonium and 87 are lead. How long do you think it has been since that layer of rock formed? Explain how you arrived at your answer.

