TG Ozone
{ GSS Teacher Guide Index } { All GSS Books }

{}
Objectives [] Overview [] Assessment [] Planning [] Resources
Guides for each Chapter: 1 – 2 – 3 – 4 – 5 – 6 – 7 – 8 – 9 – 10 – 11
Index of TG Investigations
Teaching Objectives
The four goals for the unit Ozone and the objectives that support these goals are summarized on the next two pages.
Goal 1: Students realize how our health depends on a stable concentration of ozone in the stratosphere.
Objective 1A: The students will grow to appreciate the unique environment that the Earth system provides for life. The fragile “layer” of ozone in the upper atmosphere of our planet that has made it possible for life to evolve on land, and which continues to protect humans and other life on Earth from destructive ultraviolet radiation.
Objective 1B: Students can relate to the direct effects of the harm of increased ultraviolet radiation on their skin and within their eyes as stratospheric ozone decreases.
Objective 1C: Students can understand how the fragile polar environments are affected by increasing UV radiation.
Objective 1D: Students can understand that plants and animals in the tropical to mid-latitude environments are affected by smaller decreases in stratospheric ozone compared to that observed at the poles.
Goal 2: Students grasp the feedback systems of the formation and destruction of stratospheric ozone.
Objective 2A: Students can explain how ozone is formed through the interaction of oxygen molecules with UV, and that this interaction is found at a specific layer in the atmosphere based on a balance between oxygen concentration and amount of UV radiation. Students can deduce that this specific formation of UV in the atmosphere created the stratosphere.
Objective 2B: Students can understand that molecules containing chlorine and bromine act as catalysts for the destruction of ozone in the stratosphere.
Objective 2C: Students can illustrate the negative feedback in which the energetic wavelengths of UV are needed to form ozone, and, the positive feedback in which UV, when combined with CFCs, is part of the catastrophic destruction of ozone.
Objective 2D: Students can discuss that different chemical species of ODSs have varying effectiveness in destroying stratospheric ozone (Ozone Depleting Potential, ODP), and eliminating the worst offenders is/was of the highest priority. The intermittent global recovery effort is to replace the worst species for destroying ozone with species with a low ODP until new chemicals are created that do not destroy ozone.
Goal 3: Students understand how we know that the loss of stratospheric ozone is connected to air pollution originating at the surface of the Earth.
Objective 3A: Students can discuss the various techniques we use to measure ozone concentration in the atmosphere, presenting the basic concepts as well as describing the strengths and weaknesses of the techniques.
Objective 3B: Students can understand that technological advances produced the loss of stratospheric ozone, but that improving science and technology discovered the problem and are being incorporated into the global efforts to recover stratospheric ozone.
Objective 3C: Students can describe the efforts being made by world governments and by the United States government to recover stratospheric ozone, and the results that have been observed up to this point in time.
Goal 4: Students realize that ozone has a good side and a bad side: the good side is the presence of ozone in the stratosphere, the bad side is the presence of ozone in the troposphere.
Objective 4A: Students can discuss the mechanism and the conditions needed produce ozone from specific air pollutants since ozone is not a direct substance humans produce.
Objective 4B: Students can relate that ozone is a health hazard to plants and animals because it is so chemically reactive when it comes into contact with sensitive tissue such as the cells lining the lungs and those within plants’ stomata.
Objective 4C: Students can describe efforts being made by the United States government to reduce the formation of ozone in the troposphere and how the development of cleaner technologies must compensate for the demands of an increasing population.
Objectives [] Overview [] Assessment [] Planning [] Resources
Guides for each Chapter: 1 – 2 – 3 – 4 – 5 – 6 – 7 – 8 – 9 – 10 – 11
Index of TG Investigations
Overview
Ozone addresses two key environmental challenges: the loss of stratospheric ozone and the increase of tropospheric ozone, both due to human-generated air pollution and both potentially deadly. While both of these topics involve complicated atmospheric chemistry, we have kept the detailed chemistry to a minimum while focusing on the discovery of, the mechanisms causing, the health problems resulting from, and what has been and still needs to be done to eliminate these problems.
The guide begins with the observance of the strange loss of stratospheric ozone over the South Pole in 1985, and the resulting efforts to understand why this was happening. Once the stage has been set to understand this mystery, Chapter 2 introduces ozone on a molecular scale, how it interacts with ultraviolet light, and the feedback systems involved in the formation and destruction of ozone. To put the mechanisms of ozone formation in historical context, the development of the stratospheric ozone layer as the Earth’s atmosphere increased in oxygen concentration 2-3 billion years ago is discussed. Chapter 3 ties the loss of stratospheric ozone to the health of living organisms: humans, animals, and plants. Two investigations allow students to determine the effectiveness of protecting ourselves from UV, and another helps students quantify how UV affects the health of plants.
Chapters 4 through 7 introduce the chemistry of stratospheric ozone loss through the historical journey through the creation of the problem and the steps involved in the discovery and identification of the problem. Chapter 4 introduces the discovery of chlorofluorocarbons (CFCs) and the rapid incorporation into industry. The inclusion of other Ozone Depleting Substances (ODPs) is discussed to stress that as technology creates new chemical species, the public must be ever vigilant on monitoring how they affect our atmosphere. Chapter 5 shows that as the technology of air quality measurements became more sensitive, questions soon arose to the effect of an apparently safe chemical that did not readily breakdown in our atmosphere. If it did not breakdown near the surface of the Earth as many of our air pollutants, were CFCs stable in other levels of our atmosphere where different conditions exist? Chapter 6 takes us to the “Surprise of ’85” in which hypotheses of CFCs on ozone are discussed. Chapter 7 illustrates which hypothesis was verified through research expeditions.
Since the dramatic effects of CFCs on stratospheric ozone was verified by advancing technology of measuring equipment, it is critical to understand the strengths and weaknesses of the many techniques used to measure stratospheric ozone so students can best assess global recovery efforts. Chapter 8 introduces the techniques employed to measure ozone. Chapter 9 then discusses the global efforts to recover ozone.
The last two chapters deal with ozone accumulating in the troposphere, which involves the air people breath. While the presence of ozone in the stratosphere is critical for healthy life on Earth, Chapter 10 shows how tropospheric ozone is formed as a result of chemical reactions of several forms of human air pollution. Chapter 11 illustrates the health effects of breathing ozone, a highly reactive chemical that is best found in the stratosphere, and the effects on our climate.
The importance of ozone and the fundamental chemical reactions involved in the production and destruction of both stratospheric and tropospheric molecules is embodied in Science Education Standards:
Chemical reactions occur all around us, for example in health care, cooking, cosmetics, and automobiles. Complex chemical reactions involving carbon-based molecules take place constantly in every cell in our bodies.
Chemical reactions may release or consume energy. Some reactions such as the burning of fossil fuels release large amounts of energy by losing heat and by emitting light. Light can initiate many chemical reactions such as photosynthesis and the evolution of urban smog.
A large number of important reactions involve the transfer of either electrons (oxidation/reduction reactions) or hydrogen ions (acid/base reactions) between reacting ions, molecules, or atoms. In other reactions, chemical bonds are broken by heat or light to form very reactive radicals with electrons ready to form new bonds. Radical reactions control many processes such as the presence of ozone and greenhouse gases in the atmosphere, burning and processing of fossil fuels, the formation of polymers, and explosions.
Chemical reactions can take place in time periods ranging from the few femtoseconds (10-15 seconds) required for an atom to move a fraction of a chemical bond distance to geologic time scales of billions of years. Reaction rates depend on how often the reacting atoms and molecules encounter one another, on the temperature, and on the properties—including shape—of the reacting species.
Catalysts, such as metal surfaces, accelerate chemical reactions. Chemical reactions in living systems are catalyzed by protein molecules called enzymes.”
Objectives [] Overview [] Assessment [] Planning [] Resources
Guides for each Chapter: 1 – 2 – 3 – 4 – 5 – 6 – 7 – 8 – 9 – 10 – 11
Index of TG Investigations
Planning Your GSS Course
Global Systems Science is intended to be an inquiry-based course with many hands-on laboratory activities and interactive discussions; but the extent to which it actually is based on inquiry depends on you!
The student book, Ozone, contains 6 laboratory experiments (one in Chapter 2, four in Chapter 4, and one in Chapter 11), one data analysis activity in Chapter 6, and several brief activities in other chapters that involve students drawing on-going chemical reactions involved in the production and destruction of stratospheric ozone.
From time to time we will make suggestions for small or large group discussions, questions to encourage thinking about information that is presented, or ways to engage the students’ interests. However, your best guide will be your own intuitions about how to get the students to interact with each other, comparing and contrasting their reactions and opinions about the information in Ozone and the results of their activities.
The format of information for each chapter include a content summary, purpose of teaching, matching National Science Education Standards, and suggestions.
Providing Feedback
Please keep in mind that this Teacher’s Guide is a work in progress. If you are reading these words, you are a trial test teacher, and your input is urgently needed. Each teaching day, please annotate the guide in the wide margin with questions you asked students, how you introduced the materials, and additional activities you used. Please mark with a star (*) or some other symbol what seemed to work best in helping your students grasp the concepts, formulate opinions, develop skills, or recognize the relationship between their own actions and the enhanced destruction of stratospheric ozone or the production of tropospheric ozone. We would like to make
sure to include these in the next version of the Teacher’s Guide. Mark with an (X) ideas that did not work well, and add notes in the margins to suggest what went wrong, and how you think we should change the Teacher’s Guide.
Send your suggestions and reviews to:
gssmail@berkeley.edu
Global Systems Science
Lawrence Hall of Science
University of California
Berkeley, CA 94720-5200
Director: Alan Gould

Thank you!
— The Authors
Objectives [] Overview [] Assessment [] Planning [] Resources
Guides for each Chapter: 1 – 2 – 3 – 4 – 5 – 6 – 7 – 8 – 9 – 10 – 11
Index of TG Investigations
Assessment Tasks
On the following pages are three suggested assessment tasks: Portfolios, Questionnaires, and Concept Maps. These tasks include both traditional and nontraditional ways of assessing student progress. A Portfolio is a collection of student work selected by the students to illustrate what they’ve learned and accomplished during the entire course. The Questionnaire is a traditional way to assess students’ knowledge about what that they gained from the unit. Following is a fuller description of each of these assessment tools.
1. Portfolios
We encourage the use of portfolios as a means of providing feedback to students and to demonstrate evidence of student progress to parents. A portfolio is a display of representative work selected by the student to illustrate what he or she has learned and accomplished during the course. Students may be encouraged to revise work created earlier to reflect more recent understanding, or a commitment to high-quality work.
Suggestions for Ozone portfolios include the following:
Chapter 1—Calculations to Question 1.1
Chapter 2—Investigation: Spectrum of the Sun
Question 2.2 Extending the Ozone Dance
Sketch of Altitude of Ozone Layer over Time (companion to graph on page 15)
Chapter 3—Investigation: Just how fast is melanoma increasing?
Responses to Questions 3.1-3.4
Investigation: Sunscreens and Clothing
Investigation: Sunglasses and UV Protection
Investigation: The Effect of Increased UV on Plants
Response to Question 3.6
Chapter 4—Essay on the Global Impact of Thomas Midgley’s Contributions
Responses to Questions 4.1-4.2
Chapter 5—Response to Question 5.1
Essay: Discuss what you would have done if you were a chemical manufacturer of CFCs shortly after reading Rowland and Molina’s warning in 1975
Chapter 6—Response to Questions 6.1-6.2
Investigation: Ozone Monitoring
Chapter 7—Concept map of the factors that create the “Ozone Hole” in the Southern Hemisphere
Chapter 8—Investigation: Ozone Measuring
Chapter 9—Essay or concept map on the pros and cons of creating technology to “clean” the air of dangerous pollutants
Chapter 10—Interviews with people with an age of at least 50 years old about their memories on the air quality in the local area during their life times.
Essay on your favorite “clean” technology, describing its benefits and the hurdles it must overcome to have widespread use
Chapter 11—Investigation: Measuring Lung Capacity
Response to Questions 11.1-11.5
2. Questionnaires
While it is not important that students learn hundreds of terms and detailed facts, some factual knowledge about the key concepts are important for students to know. This knowledge can be assessed in a number of ways including a questionnaire.
The questionnaires that students’ complete before beginning the unit will help you diagnose their needs and adjust your plans accordingly. Comparing these papers to the students’ responses on the same tasks after completing the unit will allow you to determine how your students’ understanding and attitudes have changed as a result of instruction.
Download the Ozone Questionnaire.
Ozone Questionnaire
Circle all correct answers (there may be more than one)
1. Ozone in our atmosphere protects us from what deadly radiation:
a. gamma rays b. x-rays c. ultraviolet light
d. radio waves e. microwaves f. infrared light
2. The layer of protective ozone is found in which part of the atmosphere?
a. troposphere b. stratosphere c. mesosphere
d. thermosphere e. exosphere f. magnetosphere
3. The harmful radiation that passes through the depleted ozone layer causes the following health problems in humans:
a. bone loss (osteoporosis) b. cataracts c. genetic mutations
d. high blood pressure e. skin cancer f. premature aging of the skin
4. Overexposure to the radiation passing through the depleted ozone layer cause health problems in the following way:
a. equally affects people at all latitudes
b. results in immediate problems
c. effects build with each overexposure
d. affects children more than adults
e. only affects people with blonde hair and blue eyes
f. cannot pass through clothing
5. Increasing radiation reaching the ground due to decreasing stratospheric ozone affects which of the following regions?
a. North Pole b. land areas in the tropics c. oceans
d. South Pole e. any area with yearlong snow cover f. deserts
6. Molecules of ozone are made up of the following atoms:
a. carbon b. hydrogen c. oxygen
d. sulfur e. nitrogen f. sodium
7. Ozone has the following characteristics:
a. odorless b. colorless c. non-reactive with other chemicals
d. long lasting e. corrosive f. cleanses the atmosphere
8. The reactions that form ozone can be described in the following ways:
a. only produced by components of human pollution
b. always require sun light
c. have caused a steady increase in ozone over time
d. require cold temperatures of the upper atmosphere
e. produce greater concentrations at polar latitudes than toward the equator
f. release heat to the atmosphere
9. Chemicals that destroy our protective ozone layer can be described as:
a. one molecule destroys one ozone molecule
b. acts only as a catalyst in the destruction of ozone so one molecule can destroy thousands of ozone molecules
c. only one chemical destroys ozone
d. molecules containing bromine destroy fewer ozone molecules than those containing sulfur
e. are formed only by natural processes
f. highly reactive with all chemicals in the atmosphere
10. Reactions that are depleting the ozone layer:
a. eventually produce a stable level of ozone
b. occur year round under all conditions
c. depend on the presence of sun light
d. use the same wavelength of light that produced ozone in the first place
e. never occurred before human pollution increased past a threshold
f. can be in balance immediately as soon as we stop generating the specific ozone depleting chemicals into our atmosphere
11. Restoring the ozone layer began in
a. 1960s b. 1970s c. 1980s
d. 1990s e. 2000s f. hasn’t started yet
12. Plans to restore our protective ozone layer include:
a. completely banning use of all ozone depleting chemicals by 2005
b. replacing the chemicals that destroy the greatest amount of ozone first, followed by eventual ban on using all ozone depleting chemicals
c. developing technologies that will remove ozone depleting chemicals before they can begin reacting
d. gradually phasing out the use of all ozone depleting chemicals by 2020
e. destroying stockpiles of ozone depleting chemicals before they are used
f. converting ozone depleting chemicals into less harmful forms before using
13. The ozone problem can be monitored by:
a. balloons b. rockets c. lasers
d. aircraft e. satellites f. spectroscopes
14. Of the wavelengths (infrared, microwave, ultraviolet, x-ray, gamma ray, and visible) which have the following properties:
Longest wavelength? ______________
Greatest energy? _________________
15. How often does the global community meet to review the policies in place to replenish our ozone layer?
16. The Clean Air Act
a. was first enacted in the United States in the 1970s
b. will help replenish the ozone layer
c. focuses on effects of human-generated pollution
d. requires a comprehensive monitoring program
e. has been revised to keep up with changing technology and increasing population
f. includes developing cleaner technologies
The questions correspond to the objectives as follows:
| Goal | Objective | Questionnaire Number |
| 1 | 1A | 1, 2 |
| 1B | 3, 4 | |
| 1C | 5 | |
| 1D | 5 | |
| 2 | 2A | 2, 5, 8 |
| 2B | 9 | |
| 2C | 10 | |
| 2D | 11, 12 | |
| 3 | 3A | 13 |
| 3B | 12, 13 | |
| 3C | 15 | |
| 4 | 4A | 8 |
| 4B | 7 | |
| 4C | 16 |
3. Concept Maps
Asking students to create a concept map before and after the unit is one way to determine which concepts they have learned and their understanding of the connections among these concepts. If students have not had experience in concept mapping, you might want to start them out with a hand-out showing an example (master on p. 10), a general idea of what they are to map, and starting word(s) to help get them started. Once they have had experience with concept maps, they can create them on blank sheets of paper (no photocopying required).
Alternatively, they can use concept mapping software such as
- Inspiration (http://www.inspiration.com)
- Decision Explorer (http://www.banxia.com).
- CMap (http://cmap.ihmc.us/conceptmap.html – free for noncommercial use).
- Omnigraffle (http://www.omnigroup.com/applications/omnigraffle Mac OSX)
- Freemind (http://freemind.sourceforge.net/wiki/index.php/
- Main_Page – open source software for mind-mapping.)
- Microsoft Draw (comes with Microsoft Office)
Some possible key words to use: climate, evolution, gas, tectonic, plate tectonic, greatmosphere, ozone, CFC, chlorofluorocarbon, troposphere, stratosphere, chemical, reactions, pollution, polar, Antarctica, ultraviolet, UV, cancer, melanoma, carcinoma, chemistry.
Ozone—Concept Map
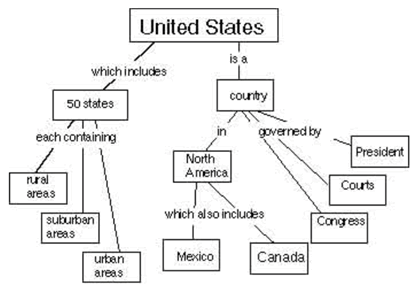
A concept map is a way of displaying your knowledge about a certain subject area. It consists of a set of words in boxes representing the most important ideas. The boxes are connected by lines and words showing how the ideas in the boxes are related. For example, at right is a concept map about the United States.
Your task is to create a concept map about the way energy is used by humans. Your concept map should show ways of thinking about how society gets and uses energy. Start with the word “Ozone” at the top. (If you’d like more space, you can draw your concept map on the back, or on another sheet of paper.)
Below are the assessment tasks for Ozone.
Download PDF of the Assessment Tasks.
Objectives [] Overview [] Assessment [] Planning [] Resources
Guides for each Chapter: 1 – 2 – 3 – 4 – 5 – 6 – 7 – 8 – 9 – 10 – 11
Index of TG Investigations
Guides for Each Chapter
The ideas presented in these Chapter-By-Chapter notes are just suggestions. From time to time we will make suggestions for small or large group discussions, questions to encourage thinking about the information that is presented, or ways to engage the students’ interests. However, your best guide will be your own intuitions about how to get the students to interact with each other, comparing and contrasting their reactions and opinions about the information in the guides and the results of the activities.
Getting to Know the Students’ Ideas
An excellent strategy for beginning any science class is to first find out what your students already know about the topic you are about to teach. What do most students understand about this subject? What misconceptions are common? Where should you start?
A suggestion is divide the class into pairs and ask them to make a list of what they think are the major problems that threaten the global environment in the world today. Emphasis should be put on consideration of the total Earth environment as distinct from regional environmental problems.
After the pairs have worked on their lists have them gather in teams of six and have them develop a prioritized list. Suggest that if the teams want to change any of their answers, they cross out, rather than erase their first answers, so that you can see what changes in their thinking have taken place.
After the groups have finished their discussions, you can hold a large class discussion so the various teams can identify points of agreement and disagreement. It is strongly recommended that you not agree or disagree with any of the students’ comments at this point. Ideas will flow more freely once the students realize that they cannot be “wrong,” at least not on the first day of class! Encourage the students to listen to each other’s arguments, and to feel free to change their minds if they think that other viewpoints are persuasive.
Getting Started
Invite students to bring in news clippings about ozone – either stratospheric or tropospheric or ozone acting as a greenhouse gas. Put these on a bulletin board, organized by topic. Encourage discussion about these articles when students bring them in, and post them on a bulletin board, organized by topic, for students to refer to throughout the unit.
Additional Investigations in this Teacher’s Guide
Ozone Theater (Dance) – chapter 2
To Tan, or Not to Tan? – chapter 3
Don’t Shine on My E. Coli – chapter 3
A CFC Deposit Law – chapter 9
How To Heal The Hole – chapter 11
Guide for Chapter 1
Strange Happenings
Content Summary
The mysterious disappearance of stratospheric ozone sets the stage for learning about ozone. The focus of the chapter is on the challenges of measuring atmospheric gases far above the surface of the Earth, the general characteristics of the layers of the atmosphere, and the basic units for measuring concentrations of atmospheric gases.
Purpose of Teaching
Although the structure of the atmosphere is a standard to be learned in grades 5-8, students now apply this knowledge toward understanding the loss of stratospheric ozone.
Earth and Space Science, CONTENT STANDARD D: (Grades 5-8) All students should develop an understanding of the structure of the earth system, in particular, that the atmosphere has different properties at different elevations.
Standards
This chapter is a review of the structure of the atmosphere, transitioning the macroscopic understandings toward a microscopic perspective of the processes that shape the structure of our atmosphere.
All students should develop an understanding of
Structure of atoms
Structure and properties of matter
Chemical reactions
Motions and forces
Conservation of energy and increase in disorder
Interactions of energy and matter
High-school students develop the ability to relate the macroscopic properties of substances that they study in grades K-8 to the microscopic structure of substances. This development in understanding requires students to move among three domains of thought—the macroscopic world of observable phenomena, the microscopic world of molecules, atoms, and subatomic particles, and the symbolic and mathematical world of chemical formulas, equations, and symbols.
Suggestions
Exploring a Mystery
Play Something’s Missing. Divide the class into small groups of 3 or 4. Each group is to make up a strange happening (something is missing i.e. all the chalk in the school is suddenly gone, or the all the school buses have disappeared.) They are to write a description of the strange happening together with all of the events, people and circumstances that supposedly caused this fanciful event. The cause(s) may be outlandish but not impossible. The events must be logically consistent and must completely explain the strange happening.
On a separate piece of paper they describe all that would be immediately observable if the strange happening had really taken place. This “evidence” is given to the rest of the class who act as investigators. They take turns asking questions of the group in order to discover what caused the event. The teacher acts as a moderator to see that the answers are consistent by using the written statements as reference.
Point out that a systematic investigation and compiling of the facts can establish the causes of strange happenings.
Layers of the atmosphere
Demonstrate natural layering by shaking a transparent tube of water pebbles, and clay. In this case gravity produces the energy that acts on the ingredients in the tube and causes the layering. The layers of the atmosphere nearer the surface of the Earth are a result of the energy of sunlight acting on the molecules of air. The temperature changes define the layers. The topmost layers are similar to those layers made in the tube of sediment – lighter materials are found near the top.
Above the mesosphere is the thermosphere where temperatures again rises due to the absorption of UV radiation by the few widely separated molecules found there. Remind the students that heat is the movement of molecules and if they are moving very fast, as they are in the thermosphere, the measurement of that motion, temperature, will have a high value.
Altitude of Peak Concentration of Ozone
The following discussion of units used to define concentration of ozone and the effects on how we observe the atmosphere may be more complicated that what you would like to teach in your class; however, it is presented if your students will be reading scientific articles on stratospheric ozone.
One of the challenges of understanding atmospheric chemistry is that the density and distribution of gases changes with altitude. Because of this, there are three common definitions of concentration used in atmospheric chemistry, each of which is necessary to observe specific characteristics of atmospheric behavior.
First, there is number density, which is the number of molecules per unit volume. This unit of concentration allows for comparison of the populations of molecules available for chemical reactions at any level of the atmosphere. i.e. this is a question of kinetics and how likely it is that molecules will collide. In this case, the peak ozone concentration is on the order of 5-6×10-12 molecules/cm3.
Another term commonly used in atmospheric chemistry is partial pressure. This is the air pressure exerted by a specific component of a gaseous mixture (such as the air) if all of the other components were removed and only the gas component of interest remained to occupy the same volume at the same temperature as the original mixture. This unit has been in used since the early 1800s when J.W. Henry proposed that the concentration of a gas dissolved in a solution is directly proportional to the partial pressure of that gas in the atmosphere above the solution. The peak partial pressure of ozone in the stratosphere ranges from 150 to 160 nanobars (nb). Compare this to the standard sea level air pressure of 1013.25 millibars (mb = 106 nb). Both the number density and the partial pressure concentrations yield similar ozone concentration vertical profiles (referred to as ozone profiles) where the peak ozone concentration is between 18 and 22 km, depending on the proximity to the equator.
Parts per million by volume (which will be shortened to ppm) is equivalent to a ratio of ozone concentration to the concentration of all atmospheric gases in a volume of space, such as a cubic liter or cubic centimeter. The advantage of this “mixing ratio” unit is that it is equivalent to mole fractions that are used in the Ideal Gas Law. The altitude of peak concentration of ozone in this unit varies between 30 and 40 km.
Why it is important to understand how atmospheric concentration units affect what you observe in the atmosphere? Notice the altitude of “peak” ozone changes depending on which concentration units is applied to the same distribution of ozone with height: number density, partial pressure, or parts per million by volume. In all three graphs, the peak concentration of ozone is found in the stratosphere.
Question 1.1
Jets tend to fly near the top of the troposphere, which is the level of the jet stream, or within the tropopause, depending on the latitude. At the poles, the troposphere is thinner due to the colder temperatures of the lower part of the atmosphere contracting those layers more than those toward the tropics.
Objectives [] Overview [] Assessment [] Planning [] Resources
Guides for each Chapter: 1 – 2 – 3 – 4 – 5 – 6 – 7 – 8 – 9 – 10 – 11
Index of TG Investigations
Guide for Chapter 2
Ozone in Nature
Content Summary
The molecular understanding of ozone: its composition and the natural production and destruction processes in the atmosphere.
Purpose of Teaching
The natural chemical behavior of ozone is essential to understand before studying the mechanisms causing catastrophic loss of the molecule in the stratosphere.
Standards
CHEMICAL REACTIONS
Chemical reactions may release or consume energy. Some reactions such as the burning of fossil fuels release large amounts of energy by losing heat and by emitting light. Light can initiate many chemical reactions such as photosynthesis and the evolution of urban smog.
A large number of important reactions involve the transfer of either electrons (oxidation/reduction reactions) or hydrogen ions (acid/base reactions) between reacting ions, molecules, or atoms. In other reactions, chemical bonds are broken by heat or light to form very reactive radicals with electrons ready to form new bonds. Radical reactions control many processes such as the presence of ozone and greenhouse gases in the atmosphere, burning and processing of fossil fuels, the formation of polymers, and explosions.
INTERACTIONS OF ENERGY AND MATTER
Waves, including sound and seismic waves, waves on water, and light waves, have energy and can transfer energy when they interact with matter.
Electromagnetic waves result when a charged object is accelerated or decelerated. Electromagnetic waves include radio waves (the longest wavelength), microwaves, infrared radiation (radiant heat), visible light, ultraviolet radiation, x-rays, and gamma rays. The energy of electromagnetic waves is carried in packets whose magnitude is inversely proportional to the wavelength.
Each kind of atom or molecule can gain or lose energy only in particular discrete amounts and thus can absorb and emit light only at wavelengths corresponding to these amounts. These wavelengths can be used to identify the substance.
Earth and Space Science: Origin and evolution of the earth system
Evidence for one-celled forms of life—the bacteria—extends back more than 3.5 billion years. The evolution of life caused dramatic changes in the composition of the earth’s atmosphere, which did not originally contain oxygen.
Suggestions
Question 2.1
Ozone is an active and, therefore, dangerous gas. It can be produced by small spark gaps such as circuit breakers. Homemade buzzers using 6-volt dry cells can produce sufficient ozone to make the gas’ presence known by its pungent smell.
Spend time allowing the students to think about the difference between dioxygen and ozone – both are made up solely of oxygen atoms but they behave so differently.
1. O2 + energy → O + O
Energy strikes the dioxygen molecule and splits it into two oxygen atoms.
2. O + O → O2
Two oxygen atoms combine to make dioxygen.
3. O + O2 → O3
One oxygen atom and a dioxygen molecule combine to make ozone.
Notice that there is no reaction in which three oxygen atoms combine to make ozone since the chances of this happening are infinitesimal.
The Energy Spectrum Investigation
The “rainbow” of visible colors change from red to yellow to green to blue to violet. Ultraviolet light is closest to the visible violet, just as infrared light is close to the visible red wavelengths.
As extensions, place two colored gels in the path of the light and describe the remaining light, or, if you have two or more light sources, mix the light with one color filter with another with a different color filter and describe the resulting light. Similarly, view different colors of paper or other objects in the light resulting from your use with the colored filters. Color filters allow transmission only of a particular color; whereas, colored objects reflect only a particular color. A red ball viewed in blue light will look black, and a red and blue gel filter will remove all light from the beam of light.
Question 2.2
This dance can be extended forever as long as there is UV of the proper wavelengths arriving from the Sun. It is important to stress that the wavelength of UV that breaks apart dioxygen molecules is different from that which breaks up ozone molecules. If the wavelengths were equal, there would be competition for the UV and the point of balance would be different than what we observe in the stratosphere. Also note that dioxygen does not have to come from the destruction of ozone, but can form without this reaction, as it must have done with the formation of the ozone layer billions of years ago.
Feedback
Feedback loops are excellent examples of systems at work. But before looking at them as systems, a common student difficulty needs to be addressed. Many students have the idea that positive means good and negative means bad. Misunderstanding the terms positive and negative can get in the way of a clear grasp of positive and negative feedback.
Feedback systems
A positive feedback is a sequence of interactions that amplifies the response to an initial change in a variable in a system. A negative feedback damps or reduces the response to an initial perturbation to the system. An example of a positive feedback would be the detonation of a nuclear bomb. Once triggered, the resulting chain reaction continues until there is no more radioactive material to be fissioned.
The classic example of a negative feedback system is the room thermostat where more heat results in less heat. A good example of a positive feedback system consists of a microphone, amplifier, and loud speaker. Sound from the speaker is amplified and sent back into the microphone only to be amplified and produce a still louder sound. If this continues the system may break down. The resulting, ear-splitting noise is referred to as “feedback.”
A system in nature that may illustrate both negative and positive feedback is the cloud/climate system. Water molecules have a high heat capacity and allow the air to continue to warm (water molecules are a greenhouse gas). Clouds, which are liquid or frozen water drops, affect both the incoming solar radiation and outgoing Earth radiation. Clouds forming at night reflect the Earth’s radiant heat back toward the ground, which warms the atmosphere. Clouds forming during the day reflect the incoming solar radiation out toward space, which cools the Earth’s surface. The presence of water vapor in the air and clouds forming predominantly at night would also create more warming – a positive feedback. If the warmer, moister air produces more clouds present during the day, more of the Sun’s energy would be reflected away from the Earth, thereby shading and cooling our planet. In this case, a warmer climate would produce a cooling effect – a negative feedback. At the present time no one can be certain the timing of cloud formation and their effect on climate change.
The Earth without Ozone
Consider the struggle for oxygen-emitting organisms to become established. Although these early organisms produced oxygen, when the concentration becomes too enriched with the gas, large portions of the algae were killed because they couldn’t live in their new atmosphere. Ultimately, this favored algae that could live in an aerobic world (oxygen-rich) to evolve. Discuss with your class whether these early episodes of massive die-offs are an example of a positive or negative feedback.
Elodea in an inverted test tube filled with water can be used to show the production of oxygen. If enough is collected, the gas can be tested by the burning splint method to show that it is oxygen.

TG-OZ2-1. Investigation: Ozone Theater
Purpose
The students physically move through the process of ozone destruction by CFCs to better help understand the process through which CFCs remove Ozone from the upper atmosphere. Students to describe to the whole group what they are doing as they go through their particular part.
Materials Needed
6 red balloons (or 12” diameter circles) made of red paper to represent oxygen, (letters may be written on the sides of all balloons to represent elements)3 green balloons to (or 12” diameter circle) to represent Chlorine,1 black balloon (or 12” diameter circle)to represent Carbon,1 blue balloon or paper circle to represent Fluorine.6 “rays” made of cardboard, 2 colored yellow and 4 colored violet or purple. 1 large yellow circle to represent the sun.1 large cardboard box to represent a discarded refrigerator with large lettering on the side saying styrofoan,(or old refrigerator or auto air conditioner, or industrial solvent) 1 sign with a clip or safety pin saying 100,000 in large black letters to put on the person playing the chlorine molecule. Enough pictures or cutouts to represent plants, animals, fungi, protists, and monerans to represent all life in the various kingdoms so that everyone in the class has a part.4 x 6 cards for each student, telling what they should say or do. (Students could make these cards out themselves as a pre-activity)
Parts are as follows:
Six students are plants which each emit an oxygen atom into the atmosphere.Six students are oxygen atoms. They first pair up to make O2 until they go to the stratosphere, where they will be broken up by UV radiation and become single Oxygens, and then recombine to make up two O3 molecules (Ozone). They will prevent the UV radiation from going to earth’s surface.Two pairs of students are light rays, one portion of which is UV(purple) and one portion of which is visible light (yellow). The yellow light is allowed to pass by the ozone and goes to earth to strike the plants, which can then emit more oxygen. The purple ray is not allowed through by the ozone. The purple ray waits until the ozone is destroyed, and then travels to earth to damage and kill the living things below.Five students are chlorofluorocarbon. The three chlorines, the carbon, and the fluorine bond together and travel to the stratosphere, where they are struck by UV light. One chlorine breaks off and becomes the free radical. This is the chlorine carrying the sign which says 100,000 times. This free radical then is able to destroy the ozone.Assorted living things from all five kingdoms. Thrive until the UV comes past the ozone. Then they begin to sicken and die.The Play
Act I:
- On a higher platform (if possible) to represent outer space, the person playing the sun emits the two yellow rays which are paired with the two purple rays. If you can go outdoors, the top of a hillside would be a good site( otherwise, students will have to use their imaginations). The rays travel to the earth’s stratosphere (halfway down the bank).
- At the same moment, all of the plants on the earth (at the bottom of the bank or hill) combine to give off the 3 pairs of oxygen molecules. Make sure that your oxygens are joined to make O2 and that they then circulate a while, slowly travelling to the stratosphere (halfway up the hill).
- When the oxygens reach the stratosphere, one sun ray, paired with its UV component, strikes ONE of the oxygen molecules, breaking it into single oxygen atoms. (The oxygen atoms become quite unhappy at being alone, and begin to seek a place to go.)
- The oxygen atoms each spot the oxygen pair, and attach themselves to make a trio, O3, which is ozone. These new ozone molecules now stretch way out as far as they can to protect the earth’s surface from the UV rays. (The UV rays try to break through, but are held back by the ozone layer (the yellow rays can go through, but the violet ones are kept back).
- Then a CFC molecule (CCl3F) made of 5 students grouped together, leaves an old refrigerator, and travels to the stratosphere to where the ozone is located.
- A UV ray strikes the CFC and releases the Cl— (now known as a free radical…the student can dress like a radical )
- The Cl— then attacks one of the ozone molecules, stripping off one oxygen atom.
- The Cl— spins off with one oxygen atom, making chlormonoxide, which then breaks apart being highly unstable, and releases the Cl— ion which then destroys the next ozone molecule, and holds up the sign, saying “I can do this up to 100 000 times!”
ACT II
- The Cl— free radical stays in the stratosphere, hunting for other ozone molecules.
- The UV rays can now travel down to the earth where they strike all of the plants and animals below, damaging them or destroying them.
The END
Followup Assignment and Assessment
Students need to sit down as soon as the play is over and working in groups, write up exactly what happened. Each group will report to the whole class and make corrections until the account is accurate. They then coach one another on the process of ozone destruction by CFCs while every student takes notes. [Suggestions to the teacher: For evaluation in a cooperative learning format, you could tell each group that one of them will be called upon to represent the group, but not which member. That person will represent the group, and they will be graded according to that person’s presentation]. Students could be asked (individually or as a group) to make a flow chart or diagram of this process or to write the process out as an assessment of their understanding. If time allows, it could be done the same day or on at a later time.
The dance could be videotaped after they get the idea and re-shown.
Objectives [] Overview [] Assessment [] Planning [] Resources
Guides for each Chapter: 1 – 2 – 3 – 4 – 5 – 6 – 7 – 8 – 9 – 10 – 11
Index of TG Investigations
Guide to Chapter 3
The Danger of UV to Living Things
Content Summary
Explore how ultraviolet light is deadly to living organisms, with special focus on humans.
Purpose of Teaching
This chapter illustrates the many ways health of living organisms is depleted with exposure to ultraviolet A and B light. Luckily, the dioxygen molecules that absorb UV-C, which is the deadliest form of UV, is not in danger of being depleted. The potential increase in the health problems caused by increasing UV is the driving force behind the global initiative to find the cause of ozone depletion and find ways to stop the causes.
We cannot separate the effects of changing life styles from decreasing stratospheric ozone in the observed trends in health problems caused by ultraviolet light. However, we can project that if we continue to have an outdoor lifestyle that does not include protection from UV, we should expect health problems to continue to grow, especially if stratospheric ozone does not recover.
Standards
Science in Personal and Social Perspectives: Personal and community health
The organizing principles for this standard do not identify specific personal and societal challenges, rather they form a set of conceptual organizers, fundamental understandings, and implied actions for most contemporary issues. The organizing principles apply to local as well as global phenomena and represent challenges that occur on scales that vary from quite short—for example, natural hazards—to very long—for example, the potential result of global changes.
Hazards and the potential for accidents exist. Regardless of the environment, the possibility of injury, illness, disability, or death may be present. Humans have a variety of mechanisms—sensory, motor, emotional, social, and technological—that can reduce and modify hazards.
The severity of disease symptoms is dependent on many factors, such as human resistance and the virulence of the disease-producing organism. Many diseases can be prevented, controlled, or cured. Some diseases, such as cancer, result from specific body dysfunctions and cannot be transmitted.
Personal choice concerning fitness and health involves multiple factors. Personal goals, peer and social pressures, ethnic and religious beliefs, and understanding of biological consequences can all influence decisions about health practices.
NATURAL RESOURCES
Human populations use resources in the environment in order to maintain and improve their existence. Natural resources have been and will continue to be used to maintain human populations.
The earth does not have infinite resources; increasing human consumption places severe stress on the natural processes that renew some resources, and it depletes those resources that cannot be renewed.
Humans use many natural systems as resources. Natural systems have the capacity to reuse waste, but that capacity is limited. Natural systems can change to an extent that exceeds the limits of organisms to adapt naturally or humans to adapt technologically.
ENVIRONMENTAL QUALITY
Natural ecosystems provide an array of basic processes that affect humans. Those processes include maintenance of the quality of the atmosphere, generation of soils, control of the hydrologic cycle, disposal of wastes, and recycling of nutrients. Humans are changing many of these basic processes, and the changes may be detrimental to humans.
Materials from human societies affect both physical and chemical cycles of the earth.
Many factors influence environmental quality. Factors that students might investigate include population growth, resource use, population distribution, overconsumption, the capacity of technology to solve problems, poverty, the role of economic, political, and religious views, and different ways humans view the earth.
NATURAL AND HUMAN-INDUCED HAZARDS
Normal adjustments of earth may be hazardous for humans. Humans live at the interface between the atmosphere driven by solar energy and the upper mantle where convection creates changes in the earth’s solid crust. As societies have grown, become stable, and come to value aspects of the environment, vulnerability to natural processes of change has increased.
Human activities can enhance potential for hazards. Acquisition of resources, urban growth, and waste disposal can accelerate rates of natural change.
Natural and human-induced hazards present the need for humans to assess potential danger and risk. Many changes in the environment designed by humans bring benefits to society, as well as cause risks. Students should understand the costs and trade-offs of various hazards—ranging from those with minor risk to a few people to major catastrophes with major risk to many people. The scale of events and the accuracy with which scientists and engineers can (and cannot) predict events are important considerations.
SCIENCE AND TECHNOLOGY IN
LOCAL, NATIONAL, AND GLOBAL CHALLENGES
Science and technology are essential social enterprises, but alone they can only indicate what can happen, not what should happen. The latter involves human decisions about the use of knowledge.
Understanding basic concepts and principles of science and technology should precede active debate about the economics, policies, politics, and ethics of various science- and technology-related challenges. However, understanding science alone will not resolve local, national, or global challenges.
Progress in science and technology can be affected by social issues and challenges. Funding priorities for specific health problems serve as examples of ways that social issues influence science and technology.
Individuals and society must decide on proposals involving new research and the introduction of new technologies into society. Decisions involve assessment of alternatives, risks, costs, and benefits and consideration of who benefits and who suffers, who pays and gains, and what the risks are and who bears them. Students should understand the appropriateness and value of basic questions—”What can happen?”—”What are the odds?”—and “How do scientists and engineers know what will happen?”
Humans have a major effect on other species. For example, the influence of humans on other organisms occurs through land use—which decreases space available to other species—and pollution—which changes the chemical composition of air, soil, and water.
Suggestions
The effect of UV energy on humans
Losing stratospheric ozone is not the direct problem to our survival; rather it is the increasing dose of deadly ultraviolet light. We shouldn’t forget about the absorption UV-C, the most energetic of the UV wavelengths, during the dissociation of dioxygen which creates ozone, which absorbs the second most deadly band of UV, UV-B.
Identifying of Melanoma
Stress that although certain skin types are more easily damaged by the Sun, resulting in increased cases of melanoma, every skin type has a chance to develop skin cancer. Students might explore the Internet to find out the reported cases of skin cancer for their type of skin coloring.
Also stress that people benefit from being exposed to the Sun in short doses. Our sleep patterns are strongly influenced by daily exposure to the Sun, as are our moods. There is a growing body of evidence suggesting that certain people are prone to depression during winter months when there is less daylight, especially in the poleward latitudes. Vitamin D, key in maintaining healthy calcium levels in the body and bones, is produced naturally by the body when the skin is exposed to the Sun. Oddly, this vitamin is not common in most foods, and it only lasts about one week in our bodies.
INVESTIGATION: Just How Fast is Melanoma Increasing?
If your students are adept at using electronic spreadsheets, spend the time to type in the data and make the plots on th eir computers.
The results appear to be a recurring pattern in our efforts to deal with many environmental problems. Despite a significant reduction in the number of deaths that result from melanoma, the increase in the percentage of cases and the increasing population contribute to an increasing number of annual deaths from melanoma each year. This suggests that the problem will not go away quickly, and that continued improvement of the detection and treatment of melanoma is needed to offset the increasing number of cases of melanoma. In the long term, people need to change their lifestyles at a young age before we see a decrease in the number and/or percent of cases of melanoma.
Questions 3.1-3.4
Question 3.1 What would you say about the difference in incidence rate of melanoma among the people of Norway compared to Italy? Although one may think of the general skin types of Italians and Norwegians, given today’s diversity found in most countries, consider the latitude of Italy and Norway – which country experiences more intense sunlight, and greater amounts of UV? What are the lifestyles of people from each country, especially during the summer? So how would the people of various skin types from each country fare?
Question 3.2 How do you think the incidence rate of melanoma might compare among Polynesians and Canadians? Similarly, as presented in 3.1, think of the latitude, intensity of sunlight, and lifestyles, and evaluate how people of different skin type fare in each country.
Question 3.3 Which group do you think would be more likely to have a higher incidence of melanoma, rural farm workers or urban office workers? Why? If the urban office worker uses the weekend to sunbathe and possibly burn, then they will be at greater risk. But in general, people exposed to the Sun, especially during the noontime hours, are more susceptible to melanoma.
Question 3.4 Would people in a high socioeconomic group be more or less likely to get melanoma than those in a lower socioeconomic one? Why? As you can see with all of these questions, no one is immune because one’s skin type, life style, amount of prevention of skin damage from the Sun, and location are the critical factors in determining one’s risk in developing skin cancer.
INVESTIGATION: Testing Sunscreens and Clothing
Just how safe are you when you take certain precautions from sun damage? There are many extensions with this investigation—brainstorm with your students to see what other factors could be tested. For example, how much does your hair protect you? What about automobile glass? Sunglasses? Do all types of glass have an equal protection against exposure to UV?
The key is for your students to develop standardized methodologies to compare various materials, and to create controlled experiments so they know how one variable is responsible for protecting them from UV. For example, if they test how different amounts of hair block UV from reaching one’s scalp, use the exact same hair in each experiment.
To extend this activity from a qualitative to a quantitative activity, have your students create an experiment that creates a series of sunprints that have been exposed to various amounts of sunlight—create this series on a cloud-free day within minutes of each other. If the increments are fine enough, detailed comparisons of shades on sunprints can be compared to the standard and a time estimate of exposure can be calculated. For example, create a series of sunprint exposures made for local noon conditions at 5-minute intervals. Don’t forget to use the acetate over the section to remove any effect from their experiments.
A more complex chemistry method of investigating sunscreen effectiveness using benzophenone is on the following pages.
INVESTIGATION: Sunglasses & UV Protection
Consider, from the first investigation in this chapter, how the number of deaths from melanoma is increasing each year despite the significant improvement of detecting and treating of melanoma. The increasing percentage of cases and the increasing US population offset the advances in public awareness and medical technology. Approach this lab in a similar way: technology of sunglasses have improved, but are they adequate to protect the eye as the pupil dilates to let in more light due to the blocking of visible light by the sunglasses.
The area of a circle is 3.14 * (pupil diameter/2)2. Because the area changes with the square of the pupil’s radius, the sunglasses, especially for lenses that block a significant amount of visible light must block a significant amount of UV.
Compare the data that is collected for the entire class to study the variation in eye sensitivity. Try to identify the factors involved in eye sensitivity to visible light. Compare the standards sunglasses manufacturers must make their lenses for the most sensitive eyes compare to the average eye sensitivity.
Finally, how does increasing UV from the loss of stratospheric ozone affect your eyes? Are lenses adequate to meet the changing conditions? If the amount of UV reaching the ground in your location increases 4% as a result of losing stratospheric ozone, your sunglasses must have a minimum threshold of that calculated earlier in the investigation plus 4%. According to the Climate Monitoring & Diagnostics Laboratory of the National Oceanic and Atmospheric Administration (NOAA) “Under cloudless conditions, each 1% reduction in stratospheric ozone results in an increase of about 1.3% in the UV- B reaching the ground.” Maps of stratospheric ozone loss can be found at: https://ozonewatch.gsfc.nasa.gov/
Factors affecting UV at ground level
If you have time to rerun the “Testing Sunscreens and Clothing”, design activities in which students can test the amount of UV reaching the ground. Run the experiments on cloudy days; or using an opaque backing, hold the sunprint paper toward the ground, testing the reflectivity of UV for various surface materials. This would be a good opportunity to have students use a method they are familiar with to design new experiments to test variables affecting the amount of UV reaching the ground. If this activity is done, stress the importance of creating controlled experiments in which only one variable is changed at a time.
It is fascinating to see how the many complex variables on Earth have helped create a world that can support living organisms. In this case, all of the deadly UV-C and most of the harmful UV-B light is blocked from reaching the ground by dioxygen and ozone molecules. These “coincidences” can be discussed in Chapter 5 when James Lovelock’s Gaia hypothesis is introduced.
Effect of UV on other living organisms
It is quite easy to become worried about environmental problems when they directly affect our health and/or our survival. But we do need to consider the effects of increasing UV on living organisms other than humans since these problems will ultimately affect our quality of life at a minimum.
As you discuss how UV harms life in the oceans and land plants, have the students relate how the loss or depletion of these organisms affects our environment and humans’ long-term quality of life.
Investigation: The effect of increased UV on plants
This experiment requires on class to set up the experiment, at least one to two weeks to let the experiment run, and a class of data collection and analysis. It may be difficult to plan this in to your class time, in which case, discuss how to best set up the experiment and discuss anticipate results.
As a minimum, students need to test the increase of UV on plants that are still maintaining the normal levels of visible light that is used for photosynthesis. This requires at 4 plants of the same species. Students may design additional tests to study the effect of increased UV on different species of plants, or study the effects of UV alone on plants (not a likely scenario on Earth, but an interesting experiment).
Prior to completing the set up of the experiment, have students review their procedures to examine if all variables are held constant except for the amount of UV. In particular, the amount of water and plant food must be held constant, either by providing equal doses on a regular feeding schedule or by testing the soil moisture and keeping these levels equal. Note that these are not equivalent, but do provide a control in the experiment. Have the students decide which is best for their experiments.
Being safe
Discuss how taking precautions for long-term skin damage compares with taking global precautions to prevent environmental problems. What level of precaution do you take for both issues?

TG-OZ3-1. Investigation:
To Tan, or Not to Tan?
by Gayle Brickert-Albrecht, Tucson High MS, Tucson, AZ
Phyllis Hoar, G.H. Braddock Sr High School, Miami, FL
Objectives
There are numerous products available which claim to offer protection from the sun’s harmful ultraviolet radiation. Creams, lotions, or gels sold for this purpose list a sun protection factor, or SPF, to indicate their relative ability to screen out harmful ultraviolet radiation. How well do sunscreens really work? Are the SPF ratings accurate? Would a regular lotion that makes no SPF claim offer any protection?
The purpose of this experiment is to determine how effective sunscreens are at filtering out ultraviolet radiation. Experiment teams compare actual product effectiveness to manufactures’ claims using benzophenone which is sensitive to the ultraviolet wavelengths that the skin. Benzophenone is a chemical which crystallizes out of solution when exposed to UV light.
If the students are practiced in using molar concentrations, you may wish to leave them to discover a method of data analysis on their own.
Materials
- samples of various sunscreens and skin lotions
- glass petri dishes with covers, one for each sample
- china marker, or tape
- balance
- spatula
- gravity filtering apparatus (funnel, support stand, filter paper)
- graduated cylinders, 250 mL and 50 mL
- 250 mL beaker
- 100 mL beaker
- wash bottle
- benzophenone
- isopropanol
Preparation
Sunscreens of varying SPF, varying media (lotion, gel, etc.), varying specialties (waterproof, perspiration-proof, hypoalergenic, etc.) may be tested, as well as products with different intents (non-SPF rated moisturizing lotions, diaper rash cream, etc.).
Since sunscreens are fairly expensive, check local dermatology centers or health organizations, such as the American Cancer Society, for the availability of free sample packets. If cost is a factor, the samples offered can be limited to 2 or 3 without diminishing the objective of the activity.
The solution is approximately 0.1 M benzophenone in isopropanol. The specific concentration is not crucial to the results of this activity. However, it is important to know the actual concentration of the prepared solution since it will affect the calculations and resulting conclusions. If the less expensive 70% alcohol is used, this solution is near the solubility limit, and takes considerable stirring to achieve dissolution. You may wish to prepare this solution in advance for the entire class. A solution of 36 g benzophenone dissolved in isopropanol to equal 1800 mL of total solution would be sufficient for a class of 24 students working in pairs testing 4 lotions. It is most conveniently prepared using a magnetic stir plate and a stoppered flask. Do not heat the mixture.
If petri dishes are unavailable, stoppered test tubes, baby food jars, or any similar container may be substituted. Be sure the container can be sealed to prevent evaporation of the solvent, and that any transparent surface exposed to the sun is coated with the sample being tested. Plastic containers are not recommended since some plastics are affected by alcohols and ketones.
Benzophenone, (C6H5)2CO, also known as diphenyl ketone or benzoyl benzene, poses a moderate health hazard—avoid contact with this substance. It is insoluble in water, but is soluble in most alcohols. It is commonly available from chemical supply companies at approximately $20 per 250 grams.
Isopropanol, C3H7OH, also known as isopropyl alcohol, is toxic and flammable. Care should be taken in its handling, and there should be no open flame in the laboratory when it is being used. Although laboratory grade isopropanol is available from any chemical supply company, 70% isopropyl alcohol will suffice for this experiment, and can be readily purchased at drug and grocery stores.
Check local guidelines for the proper disposal of benzophenone and isopropanol.
Procedure
A pre-lab discussion of the pros and cons of tanning can raise the students’ interest in the effectiveness of sunscreens for limiting exposure to UV radiation. Depending on customs and lifestyles in your community, you may wish to draw on past and current trends in advertising and other media concerning the desirability of having a “deep, dark tan” versus the dangers of melanoma and premature aging of the skin.
Each experiment team must determine what variable they are testing, and how they should select their samples in order to accomplish this.
PART I
- Prepare the light sensitive solution in a location away from direct sunlight. Combine 3.0 g of benzophenone and 150 mL of isopropanol in the 250 mL beaker. Stir the mixture until the solid is entirely dissolved. If this solution is not used immediately, it must be stored in an opaque container to avoid exposure to light.
- Using the china marker or tape, number each petri dish on the edge of its lid (the slightly larger half). Apply an even coat of one of the samples to be tested to the top outside surface of the lid. Record the sample information on the data chart next to the number of the petri dish. Care should be taken to apply the sunscreen with as uniform a thickness as possible. Brainstorm and try out some different strategies for accomplishing this. The sunscreen could be dispensed in measured volumes from a disposable syringe, or perhaps weighed out on pre-weighed petri dish tops. But the important variable is coating thickness, not total quantity. One idea is using a sheet of acetate with an extra layer of acetate in border strips on three sides. A bead of sunscreen is put near one border strip and a straight edge is used to spread the cream, with the result being the thickness of the border strips.
- Place the petri dish bottoms in a location where they will be exposed to direct sunlight for the length of the test. Pour 30 mL of the benzophenone solution into each dish and cover with the prepared lid. Record the starting time of the test, and note your observations of the solution. The dishes should be left undisturbed in direct sunlight for 2 to 4 hours. They may be left overnight.
PART II
- After the exposure time is completed, record your observations of each test. For each petri dish, use a pencil to label a piece of filter paper with the corresponding number. Find the mass of each piece of filter paper, and record it in the data chart.
- Assemble a filtering apparatus, securing a funnel over a beaker, and place the prepared filter paper in the funnel. Use a clean spatula to carefully transfer all the contents of the petri dish with the same number to the filter paper-lined funnel. With a clean dropper pipet, use the collected filtrate to wash the last of the solid material from the dish into the filter paper. Using the filtrate (the alcohol that has just run through the filter), minimizes the chances of redissolving any of the collected crystals. Take care in transferring the solid, since the mass of solid ultimately obtained represents the value of the dependent variable.
- After all of the liquid has drained out into the beaker below, carefully remove the filter paper from the funnel taking care not to lose any of the solid material. Unfold the damp paper and lay it flat on a paper towel to dry as directed by the instructor.
- When it is completely dry, find the mass of the filter paper and solid, and record this value in the data chart. Discard the filtrate and dry solid as directed by your instructor.
Results
Calculate the mass of solid formed in each test, and enter this value in the data chart. In analyzing the data, it is interesting to calculate the percent of benzophenone left in solution following exposure to UV radiation.
- First find the mass concentration of the original solution, in units of grams benzophenone/mL, by dividing the total mass of solid used by the total volume of solution made.
- Compute the mass of dissolved benzophenone present in the each petri dish by multiplying the volume in the sample by the mass concentration found in (a).
- Compute the mass of benzophenone remaining in solution after exposure by subtractiing of the dry mass collected on the filter paper.
- The percent remaining can now be calculated by using the values found in (b) and (c):
mass remaining in solution
total mass dissolved in solution
Conclusions
- Exposure to UV light causes the solid to form in the light sensitive solution. Describe the relationship between the values of “SPF” and “mass solid” for each sample in the data chart.
- Did the ability of the samples to screen out UV radiation agree with their SPF ratings?
For each sample, explain the apparent reliability of the SPF rating as observed in this experiment. - For those samples in which SPF and screening out of UV do not agree, offer an explanation of factors which could be responsible for this disagreement.
- When sunscreens are used to protect the skin from harmful UV radiation, what additional factors must be taken into consideration in judging their effectiveness?
- What other methods can be used to protect us from excessive UV radiation from the sun?
Sample DATA CHART
To Tan, or Not to Tan?
| SAMPLE DISH # | SPF/ DESCRIPTION | OBSERVATIONS PRE-TEST | OBSERVATIONS POST-TEST PAPER | MASS FILTER +SOLID (in g) | MASS PAPER (in g) | MASS SOLID (in g) |
Additional Background
Electromagnetic radiation with a wavelength between 200 and 400 nm is what is commonly referred to as ultraviolet. This range is further divided into three categories. UV-C, the shortest wavelength in this range, from 200 to 290 nm, cannot penetrate earth’s atmosphere. This type of UV light has germicidal capabilties, and is used in ultraviolet sterilizers. Exposure to the wavelengths from 290 to 320 nm, called UV-B, can cause burning of the skin, increased production of melanin in the skin known as tanning, and DNA damage associated with skin cancer. Stratospheric ozone in the earth’s atmosphere also prevents much of this radiation from reaching us. Exposure to the longest wavelengths, 320 to 400 nm, which are called UV-A, can cause eye damage such as cataracts and retinitis, and have been linked to increased incidence of melanoma. Our atmosphere offers only minimal protection from UV-A radiation.
Studies by epidemiologists show that skin cancer rates increase with greater exposure to UV light, but the actual nature of the relationship has not been clearly established. Due to stratospheric ozone depletion, the UV-B reaching the earth’s surface has risen by 4-25% from 1979 to 1989, with the largest increases occurring closer to the poles. One EPA estimate predicts 200,000 skin cancer deaths in the U.S. by 2050.
Effective sunscreens prevent both skin damage and tanning. In the U.S., sunscreens are regulated by the FDA as over-the-counter drugs, and are designed to filter out UV-B radiation. Most of these products rely on an active ingredient which includes a benzene ring, such as para-amino benzoic acid (PABA). The benzene ring absorbs UV-B photons and spreads their energy among all six chemical bonds in the ring, converting it into harmless heat. However, recent studies suggest that even small amounts of UV-A radiation can damage the skin’s immune system, and are connected to melanoma. For years, European and Australian sunscreens have been formulated to defend against UV-A radiation, but the FDA doesn’t accept the research of other countries. U.S. manufacturers must invest millions of dollars and thousands of hours to prove to the FDA that the formulation of their products is safe and effective. Several substances which appear promising are under study for approval in the U.S.:
- a group of benzylidene camphor derivatives which are less likely to clog pores and pose no threat to sensitive skin,
- PEG-25 PABA which is longer-lasting and less sensitizing than the old PABA,
- gamma oryzanol, derived from rice bran oil,
- jugalone, a skin tinter found in walnuts that offers broad spectrum protection.
References
- “Beach Bummer,” Mother Jones, May/June 1993, pp. 33-36.
- Chemistry in the Community, pilot edition II, pp. 162-163.
- Consumer Science. Evaluating the Effectiveness of Sunscreens. Kemtec Educational Corp., 1982. (Carolina Biological Supply Company Product Number 84-1147)
- “Into every life some UV must fall,” Science News, vol. ____, p. 61, July 23, 1994.
- National Center for Atmospheric Research news release, Nov. 1993.
- Pavia, Donald L., Lampman, Gary M., Kriz, George S., “Experiment 52: Photo reduction of benzophenone,” Introduction to Organic Laboratory Techniques, 3rd Ed., Saunders College Publishing, Philadelphia, 1988. ISBN 0-03-014813-8.
- Williamson, Kenneth L., “Photochemistry. The Synthesis of Benzopinacol,” Macroscale and Microscale Organic Experiments, 2nd Ed., D.C. Heath … Co, Lexington, MA, pp. 586-593, 1994. ISBN 0-669-24346-9.

TG-OZ3-2. Investigation:
Don’t Shine on My E. Coli
by “Skip” Zwanzig, edited by Eloise Farmer
If the school is equipped with material to safely use and dispose of microbial cultures, this may be used by a class to show the effects of UV radiation on living organisms.
Background
Students should already have studied the structure and function of DNA, and should have previous knowledge of the structure and functions of enzymes.
Introduction
For an organism to continue its species from generation to generation, it must be able to correctly pass on hereditary information which is carried by its DNA. The sequence of bases in DNA carries the information necessary for the life functions which promote the survival of the organism. DNA can be damaged by either natural or man-made environmental factors. When this damage occurs and goes uncorrected, the DNA cannot pass on correct information, resulting in malfunction, mutation, or death of an organism. Enzymes found in the cell can often repair damage, which allows the cell to function properly.
Molecules of DNA are shaped like spiral staircases, with the sides of the stair made of deoxyribose sugar connected by phosphate molecules. The “rungs” of the staircase are made of four bases: adenine (A), guanine(G), cytosine(C), or thymine(T). These bases pair up only as TA or AT, and CG or GC. The difference between one organism and another is the sequence of these base pairs, and this sequence is passed on to its offspring.
A common agent in the environment which can induce DNA damage is the ultraviolet radiation (UV) in natural sunlight. Remember your last sunburn? The UV radiation caused so much damage to the cells and their DNA that the normal DNA repair mechanisms were unable to repair the damaged cells so that they died and you saw them peel away.
When UV radiation strikes DNA, it causes thymines(T) or cytosines(C) sitting next to one another to link together (remember, T is only supposed to link to A, and C with G!) This linkage, called dimerization, produces a distortion in the DNA helix. If the normal repair processes fail to remove this distortion, the cell either dies, mutates or becomes cancerous. Some humans lack the cell’s natural ability to repair this type of damage and must avoid exposure to sunlight. One form of this diability is the genetic disease cassed Zeroderma Pigmentosum (XP). A person with this disease lacks the proper genes that code for repair of this type of DNA damage and XP patients are extremely susceptible to sunlight induced skin cancers.
When a cell’s DNA is damaged by UV radiation, the DNA repair systems are activated. This is part of the cell’s defense against damage and is called the cell’s “SOS system”. In the bacterium E. coli, the organism in which DNA has been most thoroughly studied, there is a set of repair and survival-promoting genes that are repressed under normal conditions by a repressor protein called the lexA protein and promoted by the action of another protein called the recA protein.
These both act in response to DNA damage. Once DNA damage occurs, the interaction of these regulating proteins results in activation of genes that code for DNA repair enzymes.
Purpose
In the following lab activity we are going to simulate the human disease SP using UV-repair deficient bacteria. We will be using the following strains of E. coli:
- Wild type(w.t.) This is a wild type of bacteria that should grow under normal exposure to sunlight and UV light sources.
- Uvr-mutant. This strain fails to recognize and remove sites where DNA damage has occured and is the direct analof of the human disease XP.
- LexA-uvr-mutant. In addition to the defect listed in strain 2 above, this bacterium also ha a mutation that codes for the “SOS” repressor protein. This repressor must be cleaved so that the repair processes can be activated. In the mutant, the repressor protein is non-cleavable so no repair response is turned on when DNA is damaged by UV radiation.
- RecA mutant. This strain lacks the recA protein, which allows the cell to use the chromosome which is homologous as a template (or pattern to follow) to repair breaks in the whole strand of DNA. The recA protein also deactivates the SOS repressor protein, turning on the cell’s emergency response to DNA damage.
There are two forms of recA mutants. The first form, recA1, is the result of a point mutation, and makes a different protein than the repair protein. The second form, recA^, is a strain that toally lacks the gene for recA protein.
The bacterial strains, recA and lexA, can be purchased from the E.coli Genetic Stock Center or can be obtained free of charge by requesting them from Dr. Priscilla Cooper, Lawrence Berkely Laboratory, Building 934, Berkeley CA 94720. It is not necessary to use all 5 strains to show differences in ability to survive UV damage. The complexity of the study is up to the individual instructor and can be modified to suit the class.
Materials
- E. coli bacterial strains
- nutrient or luria broth agar and plates
- UV light source (optional)
- inoculating loop
- timer
- autoclave or pressure cooker
- incubator (37 degrees C)
Procedure 1
(Using a UV light source).
This requires the use of a germicidal UV light source which emits at 254 nm wavelength. **Caution: DO NOT work with UV light without proper protection. Wear UV approved safety glasses and keep all skin covered to prevent damage.
- Draw 5 parallel lines divided into 5 equal increments on the outside of the bottom of a petri dish which is filled with sterile agar (these may be purchased from any biological supply house or students can make their own culture dishes using sterile techniques). Each increment represents on unit of timed UV exposure. The lines should be about 1.5 cm. apart. Label the lines to represent each bacterial strain. For example, w.t., recA, etc.(an alternative would be to use small petri dishes, each for one time interval).
- Transfer a loopful of each strain along the line, first streaking in one direction, and then taking a second loopful and streaking ON THE SAME LINE in the opposite direction.
- Once each strain has been streaked, set the petri dish aide for about 5 minutes to dry.
- Follow the same procedure for a second petri dish. This will be the control.
- Take the first petri dish, remove the cover (UV light will not penetrate the lid) and place a 3 x 5 card over the petri dish exposing only one increment of each line. Place the UV light source three meters above the petri dish (for safety reasons, this light should be shielded) and turn on the UV light for 30 seconds. Turn off the light source, move the card to the second increment and expose the bacteria for another 30 seconds. Continue this procedure until all but one increment has been exposed. Your exposure increments will then range from zero to 120 seconds.
- Incubate both plates overnight at 37 degrees C.
- Record results. Be as accurate as possible in your descriptions. You may use drawings.
Procedure 2
(Using sunlight as a UV source)
Follow the same procedure as above except for the time intervals involved. A good starting point would be to use 15 or 20 minute intervals. Since classes usually run 40 to 50 minutes, you may want to have later classes continue on. To prevent too much contamination, youwill want to cover the open petri dish with saran wrap, which only blocks out 25% of the UV light.
Procedure 3
(Using sunscreens)
An alternate or expansion of the above experiments is to test the effectiveness of sunscreens. A wide array of sunscreen types and numbers can be selected from. Once an average bacterial killing time has been determined, uncovered petri dishes can be covered with a single layr of saran wrap and carefully coated with sunscreen (see benzophenone lab for techniques.) The petri dish can then be exposed to UV light or sunlight to compare brands or to test effectiveness of SPF.
Assessment
Students could be asked to diagram the enzyme activity in a damaged cell, and to explain their diagrams, either individually or in groups.
Students could be asked to diagram the petri dishes and explain the diagrams
Students could physically act out the reactions in a damaged cell, taking the parts of the recA gene, the LexA, etc.
Students could answer the following questions:
- Scientists have discovered that the UV absorbing ozone layer is being depleted. What possible consequences, based on your experiment, could that have on living organisms?
- Tanning beds are designed to reproduce the effects of the sun in less time, using UV light. Based on your experimental results can there be any serious harm caused by the regular use of tanning beds? Explain.
- States like Arizona, New Mexico, and California are part of what is known as the sunbelt because of the large number of sunny days. How wuld you expect the ratio of skin cancer, in states like these, to compare with states having much fewer sunny days? Explain your answer.
- What effect does skin pigmentation have on the extent of UV damage?
- What selective pressure would there be for skin pigmentation on human populations living near the equator?
- In Australia, scientists have found a higher incidence of skin cancer. What could explain this?
- In California, the use of UV radiation has been suggested as a viable alternative to chlorine for water purification? Can you give a reason for this? Explain.
Objectives [] Overview [] Assessment [] Planning [] Resources
Guides for each Chapter: 1 – 2 – 3 – 4 – 5 – 6 – 7 – 8 – 9 – 10 – 11
Index of TG Investigations
Guide to Chapter 4
CFCs are Invented
Content Summary
The invention and uses of chlorofluorocarbons (CFCs), a gas thought to be safe to our environment but are at the heart of stratospheric ozone depletion.
Purpose of Teaching
In a sense, this discussion of CFCs may appear to ‘come out of thin air’, but many students may have heard of these gases. Accumulating CFCs became a global problem because of its apparent safety, its many industrial uses, and its ability to stay in the atmosphere for a long time.
Standards
Science and Technology
Understandings about science and technology:
Scientists in different disciplines ask different questions, use different methods of investigation, and accept different types of evidence to support their explanations. Many scientific investigations require the contributions of individuals from different disciplines, including engineering.
Science often advances with the introduction of new technologies. Solving technological problems often results in new scientific knowledge. New technologies often extend the current levels of scientific understanding and introduce new areas of research.
Creativity, imagination, and a good knowledge base are all required in the work of science and engineering.
Science and technology are pursued for different purposes. Scientific inquiry is driven by the desire to understand the natural world, and technological design is driven by the need to meet human needs and solve human problems. Technology, by its nature, has a more direct effect on society than science because its purpose is to solve human problems, help humans adapt, and fulfill human aspirations.
Technological solutions may create new problems. Science, by its nature, answers questions that may or may not directly influence humans. Sometimes scientific advances challenge people’s beliefs and practical explanations concerning various aspects of the world.
Technological knowledge is often not made public because of patents and the financial potential of the idea or invention. Scientific knowledge is made public through presentations at professional meetings and publications in scientific journals.
Suggestions
A remarkable compound
The cooling effect of an expanding gas can be demonstrated with any spray can. Wrap the bulb of a thermometer tightly along side the nozzle with aluminum foil and observe the reading as the gas is released. Draw a cross section diagram of a spray can showing the compressed gas propellant on top with a thin tube extending from the nozzle into the product on the bottom. (In the U. S. the propellant most often used is carbon dioxide because use of CFCs for this purpose was outlawed in the 1970s. Many foreign countries still use CFCs in their spray cans.)
Challenge the student to describe the motion of molecules as they escape through a small opening under pressure. Point out that if they speed up as they rush out that means they have added energy. Some of that added energy must have come from their immediate environment. The speeded up molecules take heat from their surroundings.
One way to help your students understand how a refrigerator works is to draw a diagram like this one and ask them to describe what happens to the molecules of refrigerant as they pass through the system.
Thomas C. Midgley Jr.
In one sense, two of Thomas Midgley’s inventions revolutionized global economies while changing our quality of life: leaded fuels and CFCs. Without these inventions, our environmental quality would have been devastated quite quickly if the then-existing technology were used globally; however, there were still environmental downsides to this “cleaner” technology.
Thomas Midgley was a dreamer that believed technology would make the quality of life better for everyone, but have your students debate what a “better” quality of life means to them. Is it being able to get from one place to another most quickly, or being able to communicate with anyone on the globe in a matter of seconds? Or is it to be comfortable from the elements while having an ample food supply or are “creature” comforts most important? An open discussion should reveal a wide range of thoughts and beliefs in your classroom.
Can your students identify a current person in the news who professes a similar belief that technology is the future and with it, our lives will be better? Review the newspapers and magazines during a class and discuss your findings. Bring in a range of media, some dealing with medicine, agriculture, industry, communications, etc.
What is a CFC?
The amount of chlorine in each type of CFC is important when relating to the ozone depleting potential that is discussed in Chapter 5.
Answers to
Question 4.1:
CFC-12 has 1 carbon, 0 hydrogen, 2 fluorine, and 2 chlorine atoms.
CFC-13 has 1 carbon, 0 hydrogen, 3 fluorine, and 1 chlorine atoms.
CFC-114 has 2 carbon, 0 hydrogen, 4 fluorine, and 2 chlorine atoms.
CFC-115 has 2 carbon, 0 hydrogen, 5 fluorine, and 1 chlorine atoms.
Answers to
Question 4.2:
CFC-113 = C2F3Cl3
CFC-114 = C2F4Cl2
CFC-115 = C2F5Cl1
Making CFCs
Ask the students how many things they know of that have been created through accidents or unexpected results. Microwave ovens in our kitchens came about when a scientist working with microwave radar systems noticed the chocolate bar in his shirt pocket melted when the instrument’s power was turned on and he was close to the microwave output. Likewise, ask students what technological advances came about through consistent, expected progress of an idea.
New Uses for CFCs
CFCs are really rather remarkable materials, especially given that they are made with rather toxic/dangerous materials. First is that they are an excellent refrigerant, second, they don’t readily combine with other chemicals, and third, they are not directly harmful to people or other living organisms. It really is little wonder that so many industries found uses for these chemicals.
Bring the yellow pages of the telephone book to class. Look under refrigeration. Have each student select a different company to interview. Brainstorm in class questions concerning CFCs for equipment manufactures and dealers, and other questions for service companies. Agree on a uniform series of questions for each group of businesses. After the interviews compile the answers to the questions and discuss the problem of doing without CFCs.
Invite a representative from refrigeration or cooling company to class to discuss the problem of CFC substitution.
Objectives [] Overview [] Assessment [] Planning [] Resources
Guides for each Chapter: 1 – 2 – 3 – 4 – 5 – 6 – 7 – 8 – 9 – 10 – 11
Index of TG Investigations
Chapter 5 A Mystery Solved
Content Summary
The advancement of technology enabled the observation that CFCs were accumulating in the atmosphere because so little was being destroyed by chemical reactions. Scientists then realized that these gases would be able to rise into the stratosphere, where they could break down under available UV light. The freed chlorine would be able to destroy ozone in the stratosphere. This alerted the scientific community to begin detailed monitoring of the concentrations within the ozone layer as well as begin to eliminate CFC production and use.
Purpose of Teaching
This chapter ties together the basic concepts of the earlier chapters: the ozone layer and the processes that naturally maintain it, the importance of the ozone layer in protecting living organisms from the Sun’s UV, and the global use and production of CFCs.
Standards
Science As Inquiry.
DEVELOPING STUDENT ABILITIES AND UNDERSTANDING
In grades 9-12, students should develop sophistication in their abilities and understanding of scientific inquiry. Students can understand that experiments are guided by concepts and are performed to test ideas. Some students still have trouble with variables and controlled experiments. Further, students often have trouble dealing with data that seem anomalous and in proposing explanations based on evidence and logic rather than on their prior beliefs about the natural world.
ABILITIES NECESSARY TO DO SCIENTIFIC INQUIRY
IDENTIFY QUESTIONS AND CONCEPTS THAT GUIDE SCIENTIFIC INVESTIGATIONS
Students should formulate a testable hypothesis and demonstrate the logical connections between the scientific concepts guiding a hypothesis and the design of an experiment. They should demonstrate appropriate procedures, a knowledge base, and conceptual understanding of scientific investigations.
DESIGN AND CONDUCT SCIENTIFIC INVESTIGATIONS
Designing and conducting a scientific investigation requires introduction to the major concepts in the area being investigated, proper equipment, safety precautions, assistance with methodological problems, recommendations for use of technologies, clarification of ideas that guide the inquiry, and scientific knowledge obtained from sources other than the actual investigation. The investigation may also require student clarification of the question, method, controls, and variables; student organization and display of data; student revision of methods and explanations; and a public presentation of the results with a critical response from peers. Regardless of the scientific investigation performed, students must use evidence, apply logic, and construct an argument for their proposed explanations.
USE TECHNOLOGY AND MATHEMATICS TO IMPROVE INVESTIGATIONS AND COMMUNICATIONS
A variety of technologies, such as hand tools, measuring instruments, and calculators, should be an integral component of scientific investigations. The use of computers for the collection, analysis, and display of data is also a part of this standard. Mathematics plays an essential role in all aspects of an inquiry. For example, measurement is used for posing questions, formulas are used for developing explanations and charts and graphs are used for communicating results.
FORMULATE AND REVISE SCIENTIFIC EXPLANATIONS AND MODELS USING LOGIC AND EVIDENCE
Student inquiries should culminate in formulating an explanation or model. Models should be physical, conceptual, and mathematical. In the process of answering the questions, the students should engage in discussions and arguments that result in the revision of their explanations. These discussions should be based on scientific knowledge, the use of logic, and evidence from their investigation.
RECOGNIZE AND ANALYZE ALTERNATIVE EXPLANATIONS AND MODELS
This aspect of the standard emphasizes the critical abilities of analyzing an argument by reviewing current scientific understanding, weighing the evidence, and examining the logic so as to decide which explanations and models are best. In other words, although there may be several plausible explanations, they do not all have equal weight. Students should be able to use scientific criteria to find the preferred explanations.
COMMUNICATE AND DEFEND A SCIENTIFIC ARGUMENT
Students in school science programs should develop the abilities associated with accurate and effective communication. These include writing and following procedures, expressing concepts, reviewing information, summarizing data, using language appropriately, developing diagrams and charts, explaining statistical analysis, speaking clearly and logically, constructing a reasoned argument, and responding appropriately to critical comments.
UNDERSTANDINGS ABOUT SCIENTIFIC INQUIRY
Scientists usually inquire about how physical, living, or designed systems function. Conceptual principles and knowledge guide scientific inquiries. Historical and current scientific knowledge influence the design and interpretation of investigations and the evaluation of proposed explanations made by other scientists.
Scientists conduct investigations for a wide variety of reasons. For example, they may wish to discover new aspects of the natural world, explain recently observed phenomena, or test the conclusions of prior investigations or the predictions of current theories.
Scientists rely on technology to enhance the gathering and manipulation of data. New techniques and tools provide new evidence to guide inquiry and new methods to gather data, thereby contributing to the advance of science. The accuracy and precision of the data, and therefore the quality of the exploration, depends on the technology used.
Mathematics is essential in scientific inquiry. Mathematical tools and models guide and improve the posing of questions, gathering data, constructing explanations and communicating results.
Scientific explanations must adhere to criteria such as: a proposed explanation must be logically consistent; it must abide by the rules of evidence; it must be open to questions and possible modification; and it must be based on historical and current scientific knowledge.
Results of scientific inquiry—new knowledge and methods—emerge from different types of investigations and public communication among scientists. In communicating and defending the results of scientific inquiry, arguments must be logical and demonstrate connections between natural phenomena, investigations, and the historical body of scientific knowledge. In addition, the methods and procedures that scientists used to obtain evidence must be clearly reported to enhance opportunities for further investigation.
Suggestions
The Scientific Investigation
There is a common pattern in which the advancement in technology creates a problem that is too complicated for people to infer at the time but is ultimately discovered through improving technology. Dr. James Lovelock’s instrument opened up a new world of detection that has been critical for our improving understanding of environmental problems. Starting with DDT pollution and then CFCs, scientists are still exploring the many pollutants in our environment and how they affect humans and other living organisms. The improved technologies that monitor the Earth’s environment are one of the key elements of hope that humans may learn how to create a healthy, sustainable existence on the Earth.
Gaia Hypothesis
Given the focus of the Global Systems Science materials, discussing the concept of “What is life?” is a valuable way to tie in the other materials that have been studied in class up to that point as well as explore the variety of core beliefs in the class. In a sense, Gaia is the ultimate version of global systems science, but in this case, everything is connected in a living system rather than a nonliving system.
Rowland and Molina
The discussion between Lovelock and McCarthy, the CFC chemist from DuPont,
in the early 70s is rather remarkable. CFCs have been in large-scale industrial production since the 1940s with rapid increase in production during the early 1960s (see graphs of CFC-11 and -12 production over time on page 72 of Chapter 9). This large stockpile of CFCs in the atmosphere has not decreased during this 10+ year accumulation. When the mechanisms of ozone destruction by CFCs is introduced in the next section, relate that the destruction of CFCs in the stratosphere involves several steps that limit the quantity of CFCs being destroyed each year.
Where do the pieces go?
Question 5.1 The reactions on p 43-44 can be extended indefinitely, as students should quickly discover.
Adding the effect of CFCs on ozone molecules
Discuss with your students the change of what was a negative feedback in which ozone production and destruction was balanced to the positive feedback in which the presence of CFCs destroys ozone in an unbalanced series of chemical reactions.
Are there other common catalysts students can identify? What about a catalytic converter—how does this fit into the discussion of catalysts? The platinum and rhodium metals are used to breakdown car engine exhaust into carbon dioxide, water, and the atmospheric form of nitrogen does not directly react with the exhaust, but rather act as a catalyst during the breakdown of the exhaust fumes.
Not all CFCs are created equal
The concept of chemical lifetimes may be rather new to your students, but it is quite similar to half-lives of radioactive materials. After one half life, only 50% of the original radioactive material will be left. In the case of one chemical lifetime, the amount of the starting chemical remaining in the system is 1/e or 1/2.7182818284… After two chemical lifetimes, the amount of chemical remaining is 1/(2.71828)2 or 13.5%.
Additional ozone destroying substances
As students will quickly see from the table of ozone depleting potentials, bromine is quite hazardous to the health of stratospheric ozone.
The spray-can war — The public won, didn’t it?
The global response to the stratospheric ozone problem was remarkably quick compared to other global environmental issues. And the continued monitoring of the response of ozone has been critical for revising the global actions taken today. Before reading the next four chapters, have the students discuss their perceptions of the significance of the loss of stratospheric ozone today. If this is an open discussion, designate someone to record the consensus view of the problem today. After Chapter 9, review the students’ earlier perception of the problem and compare to current data.
Objectives [] Overview [] Assessment [] Planning [] Resources
Guides for each Chapter: 1 – 2 – 3 – 4 – 5 – 6 – 7 – 8 – 9 – 10 – 11
Index of TG Investigations
Guide to Chapter 6
The Loss of ’84 and the Surprise of ’85
Content Summary
Despite sophisticated monitoring of the concentration of global stratospheric ozone, the losses of ozone were so unexpectedly large, we almost missed observing the rapidly expanding problem.
Purpose of Teaching
The timing of this discovery compared to when scientists first predicted that there might be a problem and the global community already taking a few steps to mitigate the problem illustrates a common challenge in environmental problems. That is the concept of “momentum” of a large system. Changes today do not mean the system will respond immediately. It took decades of building up the concentration of CFCs to create these catastrophic losses, and it will take decades to recover even if all emissions of CFCs were stopped immediately. And changing the behavior of global industries also has a momentum component – they cannot respond instantly, especially if an adequate substitute is not available or is not economically feasible.
Standards
History and Nature of Science
SCIENCE AS A HUMAN ENDEAVOR
Individuals and teams have contributed and will continue to contribute to the scientific enterprise. Doing science or engineering can be as simple as an individual conducting field studies or as complex as hundreds of people working on a major scientific question or technological problem. Pursuing science as a career or as a hobby can be both fascinating and intellectually rewarding.
Scientists have ethical traditions. Scientists value peer review, truthful reporting about the methods and outcomes of investigations, and making public the results of work. Violations of such norms do occur, but scientists responsible for such violations are censured by their peers.
Scientists are influenced by societal, cultural, and personal beliefs and ways of viewing the world. Science is not separate from society but rather science is a part of society.
NATURE OF SCIENTIFIC KNOWLEDGE
Science distinguishes itself from other ways of knowing and from other bodies of knowledge through the use of empirical standards, logical arguments, and skepticism, as scientists strive for the best possible explanations about the natural world.
Scientific explanations must meet certain criteria. First and foremost, they must be consistent with experimental and observational evidence about nature, and must make accurate predictions, when appropriate, about systems being studied. They should also be logical, respect the rules of evidence, be open to criticism, report methods and procedures, and make knowledge public. Explanations on how the natural world changes based on myths, personal beliefs, religious values, mystical inspiration, superstition, or authority may be personally useful and socially relevant, but they are not scientific.
. Because all scientific ideas depend on experimental and observational confirmation, all scientific knowledge is, in principle, subject to change as new evidence becomes available. The core ideas of science such as the conservation of energy or the laws of motion have been subjected to a wide variety of confirmations and are therefore unlikely to change in the areas in which they have been tested. In areas where data or understanding are incomplete, such as the details of human evolution or questions surrounding global warming, new data may well lead to changes in current ideas or resolve current conflicts. In situations where information is still fragmentary, it is normal for scientific ideas to be incomplete, but this is also where the opportunity for making advances may be greatest.
HISTORICAL PERSPECTIVES
In history, diverse cultures have contributed scientific knowledge and technologic inventions. Modern science began to evolve rapidly in Europe several hundred years ago. During the past two centuries, it has contributed significantly to the industrialization of Western and non-Western cultures. However, other, non-European cultures have developed scientific ideas and solved human problems through technology.
Usually, changes in science occur as small modifications in extant knowledge. The daily work of science and engineering results in incremental advances in our understanding of the world and our ability to meet human needs and aspirations. Much can be learned about the internal workings of science and the nature of science from study of individual scientists, their daily work, and their efforts to advance scientific knowledge in their area of study.
Suggestions
An important point can be made about scientific research. Textbooks including books like this one, which provide information for students rarely reflect the leap into the unknown that scientists often take. Research presented after the facts have been verified seem inevitable and the connections seem obvious. To make the point that science is a probing of the unknown with less than adequate instruments the following activity is suggested.
Question 6.1. Spend time discussing what the students would do if their instruments were providing what appeared to be erroneous data. Especially if it were occurring in the harsh, bitter cold environment of Antarctica. What did the British do the first year the data appeared to be drastically different from past years? First, they sent the instrument back for a major overhaul and testing to make sure it was operating correctly. And they started to look at the pattern of data during the same time period but for different years, and they discovered a growing problem in nature, not a growing problem in the inaccuracy of their instrument.
An almost missed clue
On of the problems with collecting vast amounts of data in an automated way is how to automatically check the quality of the incoming data. Back in the mid 1980s, computer technology was much slower than today, and creating automated checks would have slowed the processing of the data. But these checks are needed. Ask your students what simple tests could have been put into place that would not have slowed the problem down significantly but would have indicated there was a problem and more study was needed by a team of scientists?
Investigation: Ozone Monitoring
Questions on Graph Interpretation
1, 3. December (or SH summer) had the highest ozone concentration.
2, 3. October (or SH spring) has the lowest ozone concentration.
4. Dramatic decrease in ozone begins in September (beginnings of SH spring) after many months of stable ozone concentrations. There is also a relatively rapid rebound in ozone levels by the end of spring and the start of summer.
5. Explore several years of data to see if this signal is present each year.
6. Most likely something to do with warming temperatures or the onset of sunlight after the dark winter months.
7. These measurements are consistent with that of the graph.
What is a Dobson Unit?
Visualizing a Dobson unit is a very abstract concept that requires an understanding of, or at least a familiarity with, the Ideal Gas Laws. One way to help students visualize this is to use a container of beads that represent the atoms and molecules within the atmosphere, but in reality, we won’t be able to gather one million beads or so. But with 1000 beads that represent the distribution of the major atmospheric gases, you can demonstrate what a Dobson unit represents.
The numbers of beads representing the major gases for a dry atmosphere are:
| Nitrogen | Oxygen | Argon | Total |
| 780 | 210 | 10 | 1000 |
For a very moist atmosphere (tropics on a very humid day):
| Nitrogen | Oxygen | Water | Argon | Total |
| 760 | 200 | 30 | 10 | 1000 |
To see the contribution of argon, pull out all 10 of the beads that represent the gas. If we did this for the entire column of atmosphere above us, we would have a fair number of beads, but compare this number to the starting 1000 beads and realize that 1000 beads represents a volume of atmosphere that is less than one-ten thousandth of a cubic micron! One cubic centimeter of air at sea level and normal room temperatures contains over 2.1×1019 atoms.

TG-OZ6-1. Investigation: The Black Box
Provide closed cardboard boxes for each group of three or four students. (Shoeboxes painted black are ideal.) Glued to the bottom in the center is a block of wood cut to the shape of a parallelepiped. The bock should be about 15 cm wide and 8 cm high. If many of your students are bright add a smaller cube of wood on top. In both long sides of the boxes are a series of holes 3-cm apart in three rows, which are also, 3 cm apart and extend along the width of the boxes.
Give each team several thin sticks that can fit in the holes. (The old game of Pick-Up-Sticks has just the right pieces.) The teams also get a centimeter ruler. Tell the students that there is something in the black box. Give no other information. Their challenge is to make a drawing of it. Explain that all teams have the same problem. The sticks and the ruler are their only measuring instruments. They should be encouraged to record their findings on paper as they discover them. A time deadline can give the investigation a sense of urgency.
After the investigations are complete have the teams present their drawings and compare them. You will be urged to open the boxes so that they can see if their drawings are “correct.” Resist the temptation to give the students this closure. The sense of uncertainty should remain with them.
The following questions may help a summing up discussion:
- What does the box represent? (Nature, the unknown)
- What do the sticks represent? (The tools, research instruments with which we investigate nature.)
- What does the ruler represent? (The agreed upon standards and rules that enable scientists all over the world to compare their findings.)
- What do the holes represent? (The restraints of our research methods. The crudeness of our instruments. The limitations of our minds. If the holes were closer together we could probe nature with finer detail.)
- And what does the structure inside the black box represent? (The truth? Reality? Point out how little they can learn with their present instruments. Does the structure inside have color? What material is it made of? What other properties remain to be discovered? (hardness, texture) Is it possible that it has properties for which we do not have a name? How could we learn more about this or any unknown?
Objectives [] Overview [] Assessment [] Planning [] Resources
Guides for each Chapter: 1 – 2 – 3 – 4 – 5 – 6 – 7 – 8 – 9 – 10 – 11
Index of TG Investigations
Guide to Chapter 7
Expedition to Antarctica
Content Summary
Explore the steps taken to identify the mechanisms of stratospheric ozone loss. This is a good illustration of the scientific process of the largest scale.
Purpose of Teaching
This chapter illustrates how hypotheses developed by experiments in the laboratory or in theory must be tested and verified within the environmental conditions of the stratosphere, in particular, during and following the harsh winter season of the Southern Hemisphere. It also introduces the value of having measurements using different techniques, since no technique ideally provides complete information concerning both time and spatial scales.
Standards
History and Nature of Science
SCIENCE AS A HUMAN ENDEAVOR
NATURE OF SCIENTIFIC KNOWLEDGE
HISTORICAL PERSPECTIVES
(For details, see Chapter 6 Standards)
Suggestions
Trying to explain the “hole”
This is a good example of science in operation – creating hypotheses to describe observations, but set up in a way to test whether feasible or not. The design of this step in the scientific method is critical in producing valuable results that advance our scientific knowledge. Without this, we might be collecting volumes and volumes of data that don’t focus on achieving testable results.
The spectroscopic record
The 1986 research in the Antarctic produced evidence that the chlorine was the culprit in rapid loss of stratospheric ozone during the early Spring. Now, the next steps in the research were focused on identifying the factors and mechanisms that caused these seasonal observations.
Why over the South Pole? Why in the Spring?
Antarctica has a unique setting that produces an environment most of us cannot envision. Some of it is due to its extremely cold, dry climate, the location at the pole which creates a season without sunshine, and that the continent is surrounded by oceans which help create the “Roaring Forties” (the stormy region of tropospheric weather extending between 40 and 50 degrees latitude, most often referring to the Southern Hemisphere). These factors create the extreme cooling and isolation needed to produce the South Polar Vortex.
It may be helpful for your students to visually experience the Antarctic by going on-line and visit several websites of research expeditions in which live and archived pictures are displayed.
Davis Station, Antarctica:
http://www.aad.gov.au/asset/webcams/davis/default.asp
Mawson Station, Antarctica:
http://www.aad.gov.au/asset/webcams/mawson/default.asp
Casey Station, Antarctica:
http://www.aad.gov.au/asset/webcams/casey/default.asp
Archived pictures:
http://www.escapeartist.com/unique_lifestyles/Antarctica.html
Ozone loss over the Arctic
Examine a globe with your students and discuss how the Arctic differs from the Antarctic and whether these geographic differences would lead to a different climate even though both are located at the poles of the planet.
Ozone loss over the United States
These losses also have a seasonal signal, occurring during the winter/spring months. Current research is attempting to identify the primary mechanism since if it were the mixing of ozone-depleted polar stratospheric air with that of the mid-latitudes, losses would limited to the early spring months. The role of sulfur-containing aerosols is being investigated as the primary mechanism. This highlights that atmospheric chemistry of the stratosphere is very complicated and that constant global monitoring is required to identify unexpected responses in the stratosphere, which leads us to Chapter 8.
Objectives [] Overview [] Assessment [] Planning [] Resources
Guides for each Chapter: 1 – 2 – 3 – 4 – 5 – 6 – 7 – 8 – 9 – 10 – 11
Index of TG Investigations
Guide to Chapter 8
Measuring Ozone
Content Summary
Examine the variety of ways to measure stratospheric ozone, and compare the strengths and weaknesses of each technique.
Purpose of Teaching
Rarely, in measuring atmospheric variables, does one type of instrument provide complete information because of the huge range in time and spatial scales involved in atmospheric processes. These considerations need to be considered in dealing with all environmental issues.
Standards
Science as Inquiry
USE TECHNOLOGY AND MATHEMATICS TO IMPROVE INVESTIGATIONS AND COMMUNICATIONS.
. A variety of technologies, such as hand tools, measuring instruments, and calculators, should be an integral component of scientific investigations. The use of computers for the collection, analysis, and display of data is also a part of this standard. Mathematics plays an essential role in all aspects of an inquiry. For example, measurement is used for posing questions, formulas are used for developing explanations, and charts and graphs are used for communicating results.
Suggestions
The connection between UV and ozone has been known since 1881 when Hartley and Cornu noted the absence of UV in the incoming solar spectrum and discovered that ozone could absorb UV. From this knowledge, there have been a wide array of instruments designed to measure the concentration of ozone by using known intensities and wavelengths of UV: Dobson spectrophotometer; LIDAR (ozone measuring versions); and photospectroscopic sensors carried on ozonesondes, rocketsondes, and aboard aircraft, satellites, and the space shuttle. There are other ways to measure ozone concentrations, but this is the most common technology employed to do this task.
At the end of the chapter, whether or not you have looked at the strengths and weakness of the many techniques used to measure ozone, discuss with your students the role of technology in creating the ozone problem in the first place. Discuss how technology was used to discover the problem, identify the mechanisms that cause the destruction of ozone, and how monitoring the global change in ozone levels is critical for the long-term recovery of stratospheric ozone.
Investigation: Ozone Measuring
This investigation requires students to collect details on the techniques used to measure ozone in the atmosphere. As a start, use the links in the appendix of the student guide.
Students could also explore other situations in which multiple techniques are used to measure particular phenomena. Discuss students’ ideas. For example, can using body temperature only monitor human health?
Resolution of Satellite Data
For interactive software programs that illustrate the effect of resolution on satellite data, see the Global Systems Science Digital Earth Watch Software, DigitalImageBasics, available on the Global Systems Science Software Download web page.
Objectives [] Overview [] Assessment [] Planning [] Resources
Guides for each Chapter: 1 – 2 – 3 – 4 – 5 – 6 – 7 – 8 – 9 – 10 – 11
Index of TG Investigations
Guide to Chapter 9
Global Efforts to Recover Ozone
Content Summary
Survey the historical development of the global efforts to restore the ozone layer, examine how the concentrations are responding over time, and consider innovative technology that may help in its recovery.
Purpose of Teaching
Many environmental issues can overwhelm people as they learn of the scale and implications of global problems. This chapter may help by providing a documented case in which humans responded globally and relatively quickly to perceived problems.
Standards
Science in Personal and Social Perspectives
Personal and Community Health
In particular, “many high-school students hold the view that science should inform society about various issues and society should set policy about what research is important. In general, students have rather simple and naive ideas about the interactions between science and society. There is some research supporting the idea that S-T-S (science, technology, and society) curriculum helps improve student understanding of various aspects of science- and technology-related societal challenges.”
ENVIRONMENTAL QUALITY
Natural ecosystems provide an array of basic processes that affect humans. Those processes include maintenance of the quality of the atmosphere, generation of soils, control of the hydrologic cycle, disposal of wastes, and recycling of nutrients. Humans are changing many of these basic processes, and the changes may be detrimental to humans.
Materials from human societies affect both physical and chemical cycles of the earth. Many factors influence environmental quality. Factors that students might investigate include population growth, resource use, population distribution, overconsumption, the capacity of technology to solve problems, poverty, the role of economic, political, and religious views, and different ways humans view the earth.
SCIENCE AND TECHNOLOGY IN LOCAL, NATIONAL, AND GLOBAL CHALLENGES
Science and technology are essential social enterprises, but alone they can only indicate what can happen, not what should happen. The latter involves human decisions about the use of knowledge.
Understanding basic concepts and principles of science and technology should precede active debate about the economics, policies, politics, and ethics of various science- and technology-related challenges. However, understanding science alone will not resolve local, national, or global challenges.
Progress in science and technology can be affected by social issues and challenges. Funding priorities for specific health problems serve as examples of ways that social issues influence science and technology.
Individuals and society must decide on proposals involving new research and the introduction of new technologies into society. Decisions involve assessment of alternatives, risks, costs, and benefits and consideration of who benefits and who suffers, who pays and gains, and what the risks are and who bears them. Students should understand the appropriateness and value of basic questions—”What can happen?”—”What are the odds?”—and “How do scientists and engineers know what will happen?”
Humans have a major effect on other species. For example, the influence of humans on other organisms occurs through land use—which decreases space available to other species—and pollution—which changes the chemical composition of air, soil, and water.
Suggestions
As pointed out at the end of Chapter 7, there are still unexplained losses of stratospheric ozone occurring over the mid-latitude regions of the Earth, and research is underway to identify the mechanisms. However, the global community acted rather quickly and conservatively to deal with the loss of stratospheric ozone. And they haven’t stopped responding to the results based on continued monitoring, which has often showed that additional restrictions must be made to our chemicals used in our environment.
Global agreements
The world governments responded very quickly to the building evidence of a potential problem involving growing CFC concentrations in the atmosphere and the interaction with stratospheric ozone. The Vienna Convention was held in March, 1985, two months before the article Joe Farman co-authored about the significant loss of stratospheric ozone over the Antarctic was published. The Vienna Convention obligated countries to respond if human activity were found to be affecting the ozone layer. As Farman’s research and Susan Solomon’s research in 1986 showed, human activity was directly responsible for altering the nature of the ozone layer. The Montreal Protocol was convened in 1987 to then discuss what actions needed to be taken based on what was known at that time.
After examining the table on page 71, have the students discuss conclusions they can make based on changing efforts to recover the health of the stratospheric ozone. In particular, note the expanding list of ozone depleting substances that are eliminated and the shortened time frame for cessation of consumption and/or production.
Response of Stratospheric Ozone
This section was intentionally designed to provide basic data since the monitoring the response of stratospheric ozone has produced a tremendous amount of data that is showing complex interrelationships with global climate change and other tropospheric pollution. In general, the responses have slowed the loss of ozone, and we may have entered the recovery phase. For current measurements of global ozone measurements, see http://toms.gsfc.nasa.gov/
Innovations
This is where “big” ideas are needed. If the class has not learned how to brainstorm, this would be an ideal time to introduce the concept. Although the students don’t have enough understanding of the chemistry and technology needed to truly create innovative methods to destroy stockpiles of ozone depleting substances or remove large amounts from the atmosphere, they can brainstorm to identify the challenges that need to be overcome. First, in the process of destroying ODPs, make sure deadly components are not generated during this process. Remember, fluorine, bromine, and chlorine are hazardous to human health. Will the students think of converting the materials to other less harmful but useful forms that can replace the current chemicals? While identifying the challenges to overcome removing ODPs already in the atmosphere, students have to face a way to collect gases distributed throughout the atmosphere, which includes high altitudes. How do you get there and stay long enough to capture large amounts of a material, and how do you dispose of it once you collect it? As an example of how “heavy” the gases could be while accumulating, each person in the United States consumes enough materials that put over two tons of carbon dioxide in the atmosphere each year.
Have the class prepare position papers and debate the topic “It is up to individuals not government to prevent global environmental disasters.”
Points to be considered:
- The actions of society are the sum of the actions of individuals.
- Only actions by governments can control international problems.
As a summary to the chapter, have the students discuss their outlook on the problem. Is the loss of stratospheric ozone still a problem? Is the global community still adequately involved if it is a problem? Is the United States doing enough to deal with the problem? The following activity is another option.

TG-OZ9-1. Investigation: A CFC Deposit Law
Role play on legislation involving Ozone Depletion
Purpose: To explore how legislation might be made to encourage people to recycle CFCs.
Overview: Students debate a proposed piece of legislation that would require a $50 deposit to be required on the sale price of any appliance that uses CFCs. The deposit would be returned when consumer presents documentation showing that CFCs have been drained at time of disposal.
Proposed legislation:
Given that the release of CFCs into the atmosphere has been strongly implicated in the depletion of the stratospheric ozone layer; and
Given that United States is a signatory of the Montreal Protocol intended to reduce release of CFC and other ozone depleting materials;
The following legislation is proposed.
A $50 CFC collection deposit shall be charged at the time of original sale on all appliances that contain CFCs. These items at present include refrigerators, freezers, central and room air conditioners, and automobile air conditioners. The deposit will be refunded to the consumer upon receipt of proof that CFCs have been removed and collected before disposal. Should appliances use non-ozone depleting materials, they would be exempt from the legislation.
Roles involved:
Legislators, appliance dealers, renter associations, representatives of retired people, homeowners group, appliance manufacturers, importers, chemical companies making alternatives to CFCs, new car dealers, used car dealers, garbage collectors, electrical appliance repair associations, representative of apartment owners, environmental group, and others as determined by teachers.
During the legislative hearing, the legislators are looking to answers to the following questions. In general, what will the people you represent most likely think about the legislation. Consider the following specific questions the legislators have.
- When should this legislation go into effect?
- Where should the deposit money be kept? How can the fund be maintained so not be become a burden on taxpayers (like the Savings and Loan problem)? Should the money be added to the cost and a pool of money maintained by the appliance industry?
- Who should be responsible for returning the deposit? How can the original purchaser receive back the deposit if appliance is sold or transferred?
- What appliances should be covered by the deposit?
- How can the program be enacted to insure that a deposit was paid at the time of purchase?
- What can be done to insure that an appliance may not be turned in twice?
- How can the program insure that CFCs are actually collected and not just vented to the atmosphere?
- Should some of the deposit money be made available for people who recycle CFCs from appliances purchased BEFORE the implementation date of this legislation? How would this grandfathering be paid for if new appliances without CFCs rapidly gain dominant market share?
- If certification of CFC removal team is desirable, who should set standards for certifying removers?
- Can the purpose of the legislation, encouragement for safe removal of CFC, be accomplished without the need of this deposit? Does the financial reward help accomplish the goal?
- Is the proposed deposit too high? too low? Will it create a market for stolen goods?
- How will administration costs be handled? What charge may be included to handle this?
As a follow up to the presentation, students might make a consequences wheel to explain what they learned in the legislative hearings. Students might try preparing a systems map explaining the connections and potential problems.
Objectives [] Overview [] Assessment [] Planning [] Resources
Guides for each Chapter: 1 – 2 – 3 – 4 – 5 – 6 – 7 – 8 – 9 – 10 – 11
Index of TG Investigations
Guide to Chapter 10
The Other Face of Ozone
Content Summary
A molecule that is vital to the origin and survival of living organisms is also quite deadly as well. Ozone is highly reactive, and quickly oxides most materials, organic or inorganic, that it comes into contact with. Prior to human generated pollution, this chemical is found in low concentrations in the troposphere; however, chemical reactions of certain pollutants create ozone in sufficient concentrations to be hazardous to humans and other life forms.
This chapter deals with how chemical reactions form ozone in the air we breathe, how we are monitoring the concentrations, and what the United States is dong to mitigate the problem.
Purpose of Teaching
Although not related to the loss of stratospheric ozone, the increasing concentration of ozone in the troposphere and the severe effects on living organisms require that the topic be included.
Standards
Science in Personal and Social Perspectives
Personal and Community Health
ENVIRONMENTAL QUALITY
SCIENCE AND TECHNOLOGY IN LOCAL, NATIONAL, AND GLOBAL CHALLENGES
(For details, see Chapter 3 Standards)
Suggestions
Interestingly, from a Gaia perspective, the Earth has placed a hazardous chemical high up in our atmosphere so it can protect living organisms from deadly UV radiation, and the effects of the chemical are far removed from living organisms as well. But humans are changing the balance by creating significant amounts of ozone in the air we breathe, while at the same time decreasing the concentration in the stratosphere, allowing greater amounts of harmful UV to reach us on the ground.
This problem is similar to CFCs destroying stratospheric ozone—the advancement of technology indirectly created an environmental hazard. In this case, ozone-bearing smog is created through a complex chemical reaction that needs specific atmospheric conditions in order to occur. CFCs would not be as much of a threat to stratospheric ozone if we didn’t have such cold temperatures at the poles over an extended period of time that allows the formation of polar stratospheric clouds. In the case of smog, there needs to be the right mix of unburned hydrocarbons and nitrogen oxides and warm, sunny days with calm winds.
Ground Level Ozone
The ozone near the ground acts as ozone in the stratosphere: it absorbs UV. Increasing concentrations of ozone near the ground masked the effects of losing stratospheric ozone in the mid-latitudes. However, since breathing ozone is harmful, we are facing a double-edged problem. We must deal with both problems (decreasing stratospheric ozone and increasing tropospheric ozone).
Volatile Liquids
Explore the volatility of various liquids by opening containers of the liquids at the back of the room and have students raise their hands when they smell a new odor.
Another experiment consists of placing equal volumes of various liquids in the same location of the classroom and record the amount of time needed to evaporate each liquid. If a video camera is available, record the time sequence.
Sources of Smog
Ask students to complete a home and/or school inventory of volatile chemicals that contribute to smog. Tally the results that include the volume of the liquids.
Smog and Weather
Experiencing smog events during the school year is not as great as that during the summer. However, since there is a greater chance students were outside during the summer, ask them to recall days they remember having milky skies. Have they noticed if the problem has been increasing or decreasing during the past few years, or if they have heard of severe smog problems in the news?
Ozone as a Purifier
Are there other “purifying” chemicals that have been misused? Is there a cleansing chemical that is completely safe to all aspects of the environment?
Clean Air Acts
Since the last revision of the Clean Air Act was completed in 1990, discuss whether these actions should be revised based on current observations, technologies, and economics, and discuss whether it is better to give these Congressional acts enough time to find out if they are working.
Measuring Ground-Level Ozone
Using the map of PAMS network (page 81), discuss whether this adequate coverage for the United States. Include discussions on whether climate change may alter long-term wind patterns, such that the upwind and downwind sites may be misplaced in 10-30 years.
Are We Making a Difference?
Examine the graphs on pages 82-83. Based on the trends, 1970 appears to be the worst for overall emissions – the year of the revised Clean Air Act. The decrease in air pollution was not constant, especially for NOx emissions. Ask the students to consider what might have caused the spikes in emissions since 1970. Exact reasons are difficult to state definitively. Rather, encourage students to debate their ideas.
Controlling the timing of emissions and Promising Innovations
Ask students to discuss the pros and cons of using timing of emissions versus relying on developing cleaner technologies. This is similar to practicing fuel conservation versus developing more fuel-efficient technologies. Timing of emissions can create improvements very quickly – and they can be long lasting improvements. Cleaner technologies are important, but it takes a period of time before they can be developed economically and incorporated to a degree as to make a change.
Objectives [] Overview [] Assessment [] Planning [] Resources
Guides for each Chapter: 1 – 2 – 3 – 4 – 5 – 6 – 7 – 8 – 9 – 10 – 11
Index of TG Investigations
Guide to Chapter 11
Hazards of Ozone in the Troposphere
Content Summary
This chapter explores how ozone affects living organisms, with a focus on human health. It also introduces a connection to another environmental problem, that of global warming, since ozone is an efficient “greenhouse gas”.
Purpose of Teaching
This chapter ends with questions about technology’s role in creating, detecting, monitoring, and combating environmental problems, which, generally, do not have straight forward cause and effect relationships.
Standards
Science in Personal and Social Perspectives
Personal and community health
NATURAL RESOURCES
ENVIRONMENTAL QUALITY
NATURAL AND HUMAN-INDUCED HAZARDS
SCIENCE AND TECHNOLOGY IN LOCAL, NATIONAL, AND GLOBAL CHALLENGES
(For details, see Chapter 3 Standards)
Suggestions
Effects of Tropospheric Ozone on Health
Ozone is a health hazard to living organisms since it quickly oxidizes most materials it comes into contact. The timing of the highest levels of ozone occurs when more people are active outdoors: during warm, sunny days. And to make the problem even worse, young people (those most susceptible to developing problems when breathing ozone enriched air) are on summer vacation and most likely playing outdoors during the day.
Investigation: Measuring Lung Capacity
Make sure that the straw and hose have a large enough inner diameter so as to not restrict blowing into the bottle. Results will be quite unreliable if the straw or hose are too small.
Ozone Levels in Your Area and Avoiding Exposure to Ozone
Although there is a system in place to both measure and forecast ozone and a system to warn the public of unhealthy conditions, do students think the public listens? Have they altered their activities during days with unhealthy ozone levels? Have they observed changes in the public’s behavior, such as delaying paving to evening hours?
Ozone and Plants
As an additional activity, have the students contact local farmers to see if they are experiencing problems with elevated ozone levels on different types of produce. Report results in class.
Ozone and Climate
When the problem of the “ozone hole” first hit the front pages of newspapers, the public had a difficult time separating it from global climate change. Environmentalists spent quite a bit of effort trying to educate the public that the two problems were separate. And, although the basic mechanisms that created the problems are different, each is affecting components of the other. Global climate change is slowing the recovery of the ozone layer, and the production of ozone in the troposphere is contributing to global warming.
Global Warming Potential
Given that ozone doesn’t last long in the atmosphere and the generation of ozone tends to occur near cities, discuss if ozone should be considered a problem in global climate change. Is it only adding to the “heat island” effect observed around cities in which the cities are noticeably warmer than the surrounding suburbs/rural areas? Consider that these heat islands alter local storm patterns, which is producing less rain over the cities and more rain downwind.
Summary of Ozone in the Troposphere
Does the short chemical lifetime of ozone in the troposphere help us forget or ignore the problem? This is different from developing the science to identify the variables and the complex chemical reactions. How much does the public perception of the problem drive the science and government policies?
Questions about Technology
Discuss the question: Is the technology solving a problem or is the technology looking for a problem to solve? If it is the latter, does this increase the chance that the technology will be used when it really isn’t needed?
Discuss the current developments in technology and predict what might be available in 10 years. Will there be new forms of transportation? Will wind power, solar cells, fuel cells, and fusion be used globally? Will we be able to harvest the energy of thunderstorms?

TG-OZ11-1. Investigation:
How To Heal The Hole
by Rene Muñoz,
National Center for Atmospheric Research, Boulder, Colorado, 7/93
BACKGROUND
The “ozone hole” and its consequences affect all living things. Everyone now knows the 1992 Antarctic ozone hole was the largest and longest-lasting one yet.
So an International Commission on the Protection of the Ozone Layer has been urgently convened to consider additional restrictions on CFCs and other ozone-depleting chemicals.
ASSIGNMENT
One way to better understand the many sides of such a complicated global problem is to role-play the issue. Your group is one of six Interest Groups asked to make an opening statement to the members of the Commission for their consideration.
As an individual, do the following:
- Add the attached information about the Interest Group you represent to what else you have read about global ozone negotiations in this chapter and elsewhere. Consider the following for this treaty amendment process:
- How quickly could proposed new treaty amendments go into effect?
- How could new controls be enforced?
- Who will pay and how?
- What should be done about countries that refuse to sign the new treaty amendments?
- Should the new CFC alternatives be substituted for the CFCs now in use?
- Discuss with the other members of your Interest Group what you want the Commissioners to take into account when they amend the treaty. Choose someone from your group to act as spokesperson and present your case.
- After each group has made its presentation (listen up!), everyone will play the role of Commissioner when you go back to your group. Taking into account the same questions you considered above, how would you answer them again, this time acting on behalf of the Commission?
- Please hand in a copy of your group’s draft of the treaty amendments proposed by the Commission. Each individual should also answer on paper the following questions:
- How did your deliberations as Commissioners go? Were they any different from your Interest Group discussions?
- In the draft of the treaty amendments that your group wrote, did it represent all six viewpoints, or just some? If just some, how did you decide what to include and exclude? How did you balance conflicting views?
- Do you think the treaty amendments will be signed by the majority of the countries represented in international ozone treaty deliberations?
- What did you learn from this discussion-negotiation process that helps you better understand how nations deliberate with each other?
*CITIZEN (mom, teacher, environmentalist):
Eloise Feddeler
“I’ve got three sets of twins—blonds and redheads. They burn in the sun like Bill and I do. I’m concerned about ozone damage to their skin and vision.”
I teach earth systems science and ecology on the high school level, and I believe that there are serious global environmental problems including ozone depletion. So I think globally and act locally—I’m head of five area environmental projects. (Whew!)”
“But now I’m reading that sunscreens may not be living up to their advertising claims. Tell me, what’s a concerned mom (and teacher and environmentalist) to do??”
VICE PRESIDENT OF THE U.S: Al Gore
(Now that Gore and President Clinton are in the White House, he can pursue his interest in environmental leadership.)
“Our future economic progress is inextricably linked to sound policies promoting protection of the environment and wise stewardship of our natural resources.”
“The central organizing principle of the post-Cold War world is the task of protecting the Earth’s environment while fostering an economic program.”
“I believe the US can create millions of new jobs by leading the environmental revolution and speeding up our efforts to manufacture and sell environmentally superior products and technologies.”
But also consider this:
On Election Day 1992, only 6% of voters identified the environment as a key issue, and many US lobbies are pressing the government to limit or repeal environmental legislation.”
*INDUSTRIAL EXECUTIVES: Mack McFarland and Tony Vogelsberg
(*Quotes are comments of two Du Pont Company executives; Du Pont is the world’s largest producer of CFCs, with 25% of the world’s CFC production)
“Du Pont’s goal is to develop options that will meet the needs of CFCs in a safe and environmentally acceptable manner while achieving a long-term reductin in atmospheric chlorine from the compounds meeting those needs.”
“But . . . a new process needs to be created, toxicity testing, material application, restructuring.
The new stuff is not just a drop-in.”
“We’re making good progress, but full commercialization is in a distant time-frame, as we need to establish performance for many years. We need an orderly transition rather than a precipitous withdrawal, to avoid serious disruption of society.”
ATMOSPHERIC CHEMISTRY SCIENTIST: Susan Solomon
(She was there, as leader of the first international ozone expedition to the South Pole, in 1986)
“[The ozone hole] is outside anything we’ve ever seen on Earth. It’s dropped off the bottom of the chart.”
“[Ozone depletion] is perhaps a question of by how much and exactly when, not a question of ‘if.’”
Plus she also knows:
• That ozone depletion is related to the current dramatic rise in skin cancer cases.
• That ozone depletion will affect plant and ocean life.
• That CFC alternatives now being developed are not necessarily the solution.
*UNITED NATIONS SECRETARY-GENERAL: Butros Butros-Galli
(He is represented in this activity as an advocate of the developing countries of the world)
He reminds us that:
The Montreal Protocol (drawn up in 1987) allows developing countries to increase their CFC consumption, within certain levels, for the next 10 years.
How can industrialized countries try to limit technological development in developing countries?—Don’t they think that the developing countries deserve the opportunity, as they have had, to develop a modern lifestyle, for the well-being and comfort of their people?
The cost of converting to CFC alternatives for these countries often makes the changeover unaffordable. The international fund set up for this just a few years ago has insufficient funds, and more countries are requesting loans. Do industrialized nations have an obligation to assist or pay for changeovers to CFC alternatives for these countries?
TALK-SHOW HOST, GADFLY, AUTHOR: Rush Limbaugh
All atmospheric researchers, Limbaugh claims, are “dunderhead alarmists” and “prophets of doom.”
There is a “scam” being perpetrated by self-interested scientists out to procure funding for their unnecessary research. . . . He says they’re saying that “with the space program winding down, we have this unusual amount of chlorine in the atmosphere [and so] we need funding.”
But, he maintains, “Mt. Pinatubo has spewed out more than 1000 times the amount of ozone-depleting chemicals than all the fluorocarbons manufactured by the wicked, diabolical, and insensitive corporations in history.”
“Ozone is still there in sufficient quantities to protect Democrats and environmentalists alike from skin cancer.”
Limbaugh sees the fact that the great majority of atmospheric researchers agree on the basic findings of ozone depletion by CFCs is only considered evidence of how widespread the conspiracy is.
Objectives [] Overview [] Assessment [] Planning [] Resources
Guides for each Chapter: 1 – 2 – 3 – 4 – 5 – 6 – 7 – 8 – 9 – 10 – 11
Index of TG Investigations
Resources for Ozone
For latest material relevant to Ozone,
see the Staying Up To Date section of the GSS website.
The list below is from the original development of GSS and is out of date and in no way exhaustive for the resources that are available for teaching ecosystem change and related topics.
VIDEOS
- “Protecting the Ozone Layer: The Search for Solutions,” a 27-minute videocassette distributed by the du Pont Chemical Company. Contact information: Mack McFarland, du Pont, Freon Products Division, B-13230, Wilmington, DE 19898; telephone 302-774-5076.
- “Ozone-The Hole Story,” video appearing on PBS stations in 1993.
SOCIETIES AND ORGANIZATIONS
- American Board of Dermatology
- American Cancer Society, local chapters
- American Meteorological Society (AMS), Boston, MA
- Climate Protection Institute, Oakland, CA
- National Cancer Institute, Washington DC
- National Center for Atmospheric Research (NCAR), Boulder, CO
- National Oceanographic and Atmospheric Organization (NOAA), many local offices
- Northern California Cancer Center
BOOKS
- Gore, Al, “Earth in the Balance: Ecology and the Human Spirit.” New York: Plume, 1993.
- Maduro, Rogelio, and Schauerhammer, Ralf, “The Holes in the Ozone Scare: The Scientific Evidence That the Sky Isn’t Falling.” Washington, DC: 21st Century Science Associates, 1992.
- Ray, Dixy Lee, “Trashing the Planet.” ??
- Weiner, Jonathan, “One Hundred Years: Shaping the Fate of Our Living Earth.” New York, Bantam Books, 1990.
BOOKLETS/JOURNAL/MAGAZINE ARTICLES
- OIES (Office for Interdisciplinary Earth Studies), “Reports to the Nation on Our Changing Planet: The Ozone Shield.” Boulder, Colorado: OIES, Fall 1992.
- OIES, “Earthquest: EOS and GLobal Change” issue (has 4-page time-line on center foldout), fall 1991.
- (Both OIES booklets available by writing OIES, UCAR, PO Box 3000, Boulder, CO 80307.)
- E: The Environmental Magazine. Published bimonthly by Earth Action Network. $3.95/copy.
- “The Ozone Backlash,” Science, v. 260, 11 June 1993, pp. 1580-1583.
- “CFC Replacement Technologies: Help Is on the Way,” R&D Magazine, December 1992, pp. 29-32.
- “Beach Bummer,” Mother Jones magazine, May/June 1993, pp. 33-34.
See also, the Student Book for many web sites, books and articles.
Objectives [] Overview [] Assessment [] Planning [] Resources
Guides for each Chapter: 1 – 2 – 3 – 4 – 5 – 6 – 7 – 8 – 9 – 10 – 11
Index of TG Investigations
Index of Teacher Guide Investigations
- TG-OZ2-1. Ozone Theater
- TG-OZ3-1. To Tan, or Not to Tan?
- TG-OZ3-2. Don’t Shine on My E. Coli
- TG-OZ6-1. The Black Box
- TG-OZ9-1. A CFC Deposit Law
- TG-OZ11-1. How To Heal The Hole
