CC2.3. Why Do Some Molecules Absorb Infrared Energy?

{ Climate Change Contents } { All GSS Books }

Carbon dioxide gas allows visible light to pass through but will absorb infrared energy. Why is that? The answer involves a concept called resonance.
If you are pushing a child on a swing, in order to get the child to swing as high as possible, you have to time your pushes just right. If the swing’s natural cycle (frequency) without pushing is one swing every second, you must push at that frequency—once per second. You must also give the push at just the right instant, when the swing is just about to go forward. That “just right” pushing frequency needed to transfer energy from one system (you) to another (the swinger) is called the resonant frequency.
A fascinating aspect of resonant frequency is that you can feel it. When you’re pushing someone on a swing, you know when you have found the resonant frequency because you can feel your energy being transferred to the swing and the person on it. Each time you push, you can see the swing go higher and higher and higher.
Gas molecules in the atmosphere resonate when they are struck by a vibrating photon of light, but the molecules are so small, it is impossible for us to feel their resonant frequencies. In this investigation you will experiment with models of molecules that are millions of times larger than the real ones. You will use these models to see what happens when they are energized with different frequencies of vibration.
The models represent four different kinds of molecules found in the atmosphere: nitrogen, oxygen, carbon dioxide, and methane. The vibrations you will produce with your hands represent different frequencies of light.
Molecules are systems composed of different kinds of atoms connected by bonds. Many different kinds of materials can be used to create models of molecules, but not all of them will allow you to experiment with resonance.
Materials
Although there are a many combinations of materials that can be used to construct model molecules for this investigation, the following work well, are inexpensive, and readily available:

- 7 flexible struts as model molecule bonds,
e.g. plastic cable ties* (or 14 chenille stems/pipe cleaners**)
it’s best to have 2 colors. - 12 polystyrene balls***
as model atoms—1.5″ (3.8 cm) diameter - Wire cutter
- Small screwdriver
- Ruler
- Watch or clock with a second hand
* Cable ties available at hardware stores. To represent flexible molecular bonds, it may also be possible to use chenille stems (pipe cleaner), strips of springy plastic, thin flexible rods, stiff wire, or springs. Wooden materials such as Tinker toys or toothpicks are not flexible enough, so don’t use them.
** If you use chenille stems, twist two of them evenly around each other four times. Pinch the ends securely so they don’t unwind). You will have 7 “bonds” when you are finished which you will cut to 12 or 15 cm lengths for your models. The 12 cm lengths represent strong bonds and the 15 cm lengths represent weak bonds. [Note: there are two illustrative videos using chenille stems in the GSS Teacher Guide for Climate Change Chapter 2 .]
*** Styrofoam balls due to their low density may not have enough mass to work with the cable ties or chenille stems/pipe cleaners. Depending on choice of “molecular bond” material, rubber balls, nuts and bolts to represent the atoms. The trick if experimenting with new materials is to match the weight of “atoms” with the stiffness/flexibility of the “bonds.” For example, in early tests, hack saw blades stuck into tennis balls gave excellent results.
What To Do
1. Construct your models following the instructions below:

- Use three 12 cm (strong) model molecule bonds
- with 2 balls.
- Nitrogen makes up 78% of the atmosphere. Molecules of nitrogen gas are composed of two atoms of nitrogen (N) connected by a strong (short) triple bond.

- Use two 12 cm (strong) model molecule bonds
with 2 balls. - Oxygen makes up about 20% of the atmosphere. Molecules of oxygen gas are composed of two atoms of oxygen (O) connected by a strong (short) double bond.
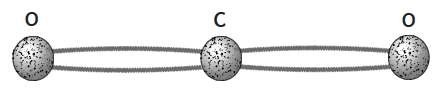
- Use four 15 cm (weak) model molecule bonds
with 3 balls. - Carbon dioxide accounts for less than one percent of the atmosphere, but it makes a very important contribution to the greenhouse effect. Carbon dioxide molecules are composed of an atom of carbon (C) in the center, connected to two atoms of oxygen (O) with a weak (long) double bond.
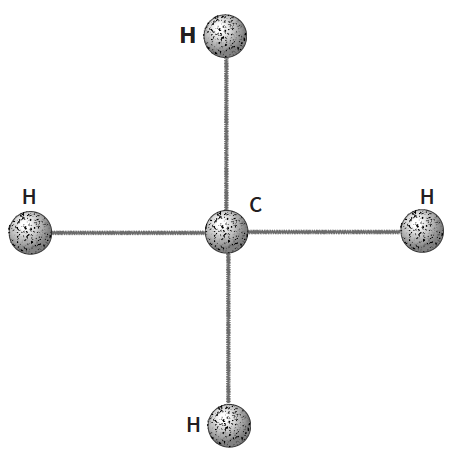
- Use four 15 cm (weak) model molecule bonds
with 5 balls. - All the atoms should be in a flat plane, as if lying on a table.
- There is even less methane in the atmosphere than carbon dioxide, but it also makes a very important contribution to the greenhouse effect. Methane molecules are composed of a single carbon atom (C) in the middle, surrounded by four atoms of hydrogen (H), at equal distances, connected by weak (long) single bonds.
- Test each model to determine its resonant frequency. For example, hold the carbon dioxide molecule by the central carbon atom and shake it up and down a few inches. Try a range of shaking speeds, or frequencies, from very slow (one shake per second) to very fast (seven or eight shakes per second).
- See if you can find a frequency at which it is much easier to keep the model vibrating. It may dance around in a rhythmic way. If it does, it has absorbed the particular frequency of energy that you put into it. This frequency is called the resonant frequency.
- Use the clock to time your shaking. Measure the resonant frequency by counting the number of vibrations in a five-second interval while your model is “dancing.” Divide by five. Do as many trials as you need until you are convinced you have measured the resonant frequency of each model or until you are convinced you cannot find one.
QUESTIONS
2.3.1 Which of the four molecules has the fastest resonant frequency?
Which has the slowest?
Which seem to have no resonant frequency?
2.3.2 If there are differences in resonant frequencies, why do you think they are different?
2.3.3 The behavior of these models are analogies to the behavior of real molecules of carbon dioxide, methane, oxygen, and nitrogen. From the observations of your models and their interactions with different frequencies of vibration, why do some gases in the atmosphere absorb infrared radiation while others don’t?
Disclaimer: The models in this investigation are adequate to explore the basic concept of a resonant frequency, but a weakness in the model is that real bonds behave differently from plastic cable ties or pipe cleaners. The oxygen double bond model using two ties and the nitrogen triple bond model using three ties are much stiffer than they should be to represent real bonds. Moreover carbon dioxide has double bonds between the carbon atom and the two oxygen atoms, but in our models they are represented by single cable ties to make it easier for the molecule models to vibrate.
In reality, oxygen and nitrogen molecules vibrate just as CO2 does. The reason O2 and N2 are not greenhouse gases and CO2 is a greenhouse gas is that in CO2 vibrations there is an asymmetric distribution of electric charge because oxygen atoms exert a stronger “pull” on the shared electrons than carbon atom does. A molecule can’t absorb light unless it has an asymmetric charge distribution. Asymmetric charge also occurs in water molecule vibrations, making water vapor an excellent greenhouse gas as well. Because oxygen and nitrogen are made of two identical atoms, they can’t generate a charge asymmetry when they vibrate.
Additional insight can be seen in this 3-minute animation from MinuteEarth: How Do Greenhouse Gases Actually Work?
This investigation is very similar to one found on the NOAA Earth System Research Laboratory, Education and Outreach site. Specifically, Earth System Interactions, Teaching Activity: What’s So Special About CO2? [Teaching Guide and Student Handouts.]
Other activities on resonance may be found at
- PhET interactives website – The Greenhouse Effect. There is a “Photon absorption” tab that reveals resonant frequencies of various heat trapping gases.
- Exploratorium website – Resonant Rings
- NOAA ESRL website – Resonance Rings

