CC3. The Composition of Earth’s Atmosphere

Chapter 3
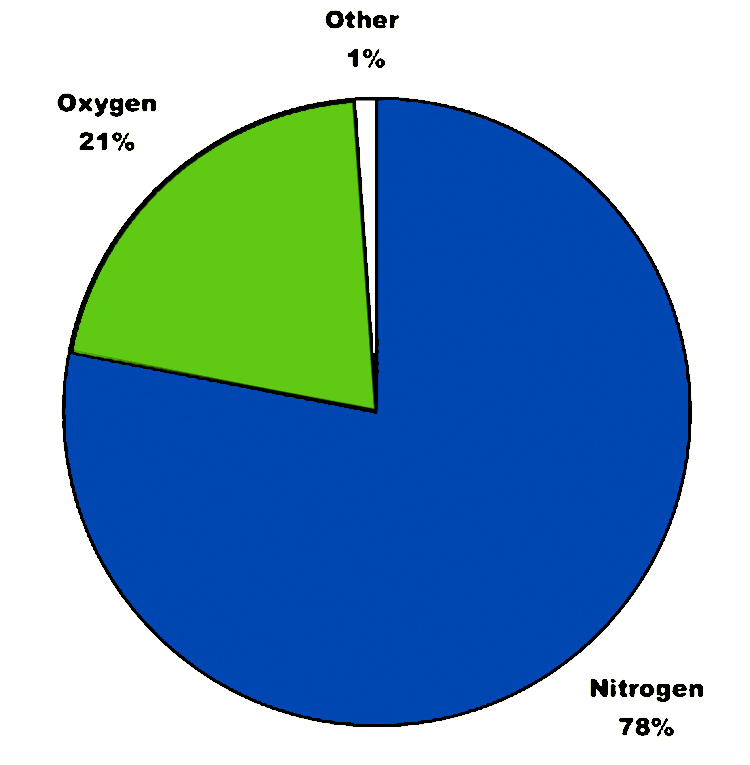
Ninety percent of the gases that make up our atmosphere are in the troposphere—the lowest and densest part of the atmosphere which extends 10–12 kilometers above the surface. The stratosphere, which extends up to about 60 kilometers, contains most of the rest of the atmospheric gases. While the very thin upper reaches of our atmosphere extend even further, it is the composition of gases in the lower part of the atmosphere that has the greatest effect on climate. These gases consist almost entirely of nitrogen and oxygen. Both of these gases are important for life, but they do not keep us warm. If our atmosphere consisted of these gases alone, our planet would be nearly as cold, and perhaps as lifeless, as Mars.

But the greenhouse gases—which make up less than 1% of the entire atmosphere—absorb heat and keep us warm. In addition to carbon dioxide and methane, the greenhouse gases include water vapor, nitrous oxide, ozone, and a family of gases called chlorofluorocarbons (CFCs for short). As described in Chapter 4, molecules of all these gases vibrate when they encounter infrared photons. They warm up, trapping heat in Earth’s atmosphere.
Measurements made at Mauna Loa in Hawaii, the South Pole, and other sites around the world show the atmosphere is changing. In the opinion of the vast majority of atmospheric scientists, this increase in the atmospheric concentration of greenhouse gases will gradually bring about widespread changes in Earth’s climate.
Although the extent, timing, and impact of these changes in various regions of the world are uncertain, the climate changes that have taken place in recent history—such as the “dust bowl” droughts that plagued the United States in the 1930s—illustrate that small changes in the average global temperature can have major and sometimes devastating impacts on life. It is therefore very important for us to have a clear picture of the sources of these gases and their relative contributions to the predicted global warming.
Composition of Gas in the atmosphere
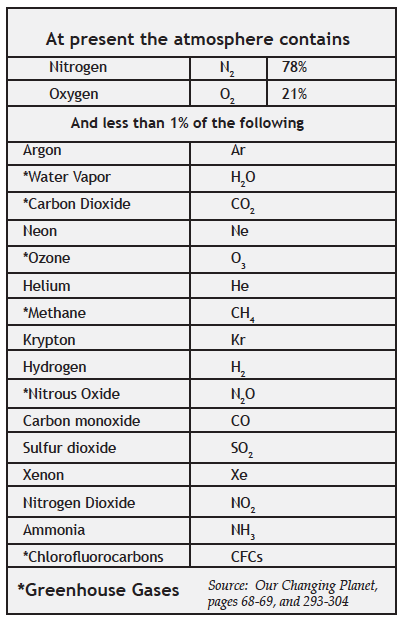
I. The Greenhouse Gases
Water Vapor (H20)
Many people believe the plume of visible steam from a boiling kettle is water vapor. However, steam and water vapor are not the same. Steam consists of large water droplets suspended in the air, while water vapor is an invisible gas. Like carbon dioxide and methane, water vapor resonates at the frequency of infrared energy, so it traps heat in the atmosphere.
The amount of water vapor in the air at any one place is highly variable. Its concentration changes hour by hour and from place to place, ranging from up to 4% in tropical rain forests, down to a few tens of parts per million in the dry, frigid air of the Antarctic.
Water vapor is produced when sunlight falls on rivers, oceans, and other bodies of liquid water. As the water warms, its molecules move faster and faster, until some finally escape, forming a gas that mixes with the air. Water vapor is also released by the leaves of plants as part of their life processes.
The concentration of water vapor is called humidity. On a warm, “sticky” day the air contains a lot of water vapor. Warmer air is able to hold a greater concentration of water vapor. That is the reason dew forms in the early morning hours, when the air cools and water vapor condenses. The water vapor reenters the atmosphere later in the morning, when sunlight warms the air and evaporates the droplets of dew.
Water vapor has always played an important role in the natural greenhouse effect. Unlike the other greenhouse gases, most water vapor is not added to the atmosphere by human activities. However, it is of special interest to scientists because its effect may change as a result of human activities. Two theories have been proposed. One suggests water vapor will increase the effect of the warming, the other suggests it will form clouds and cool Earth. At this writing, no one knows for certain which will happen.

Feedback occurs when the output of a system automatically controls the input. In the case of global warming, the output is increased global temperature.
Theory A is an example of negative feedback, in which a little warming leads to cooling, so the climate system returns to normal.
Theory B is an example of positive feedback, in which a little warming leads to more warming, so the climate system is disturbed more and more.
Carbon Dioxide (CO2)
Carbon dioxide seems to be the world’s favorite gas. It is loved by soda pop and beer drinkers. It’s bubbles put the fizz in champagne. Carbon dioxide makes baked goods “rise.” It is the most important of the greenhouse gases. Carbon dioxide has some strange properties. At low temperatures it can be compressed into a white solid we call dry ice. At room temperature, the cold solid dry ice changes back into a gas without melting into a liquid form, which is called sublimation.
Carbon dioxide bubbles out of the ground in soda springs. It explodes in vast quantities out of volcanoes. Animals and plants release it as they use oxygen for respiration. Carbon dioxide is released in forest fires. Decay of rotting plants and animals produces carbon dioxide. Without carbon dioxide to trap infrared energy, our planet would be about 33°C cooler than it is now, and it is questionable whether life ever would have evolved.
During tree growth, carbon is collected from the atmosphere and incorporated in the plant tissue we call “wood.” About half of each firewood log is composed of carbon (by weight). When the log is burned, most of the carbon is released from the wood in the form of carbon dioxide gas. James Watt’s first steam engine used wood as a fuel. The steam engine signalled the beginning of a process in which humans beings add significant amounts of carbon dioxide to the atmosphere.
Burning wood in steam engines certainly contributed to air pollution in those early days, but the amount of carbon dioxide added to the air was not that large. The carbon was simply recycled from the air to the wood, and then back again when the wood was burned. But when the most frequently used fuel was changed from wood to coal, the change in the atmosphere began in earnest. That is because coal is composed of carbon from plants that died millions of years ago. When coal is burned, it releases carbon dioxide into the atmosphere.
More and more coal was burned throughout the world in the 19th and early 20th centuries. Then people found oil, It was easier to extract from the ground, transport, and use than coal. Like coal, oil consists of the partly decayed remains of plants and animals. Today, most of the energy used to power our transportation, run our factories, heat our homes, and produce electricity comes from oil. But all of these benefits are not without costs to the environment.
Every piece of wood, every pound of coal, every gallon of oil burned releases carbon dioxide. Just one gallon of gasoline burned in a car’s engine adds about 10 kilograms of carbon in the form of carbon dioxide to the atmosphere. Each year, five tons of carbon in the form of carbon dioxide—roughly equivalent to the mass of a single large elephant—is added to the atmosphere for every man, woman, and child living in the United States.
The concentration of carbon dioxide in the atmosphere is now 25% higher than it was before the extensive forest clearing and the industrial development that began nearly 200 years ago. That percentage is currently rising at a rate of half a percent per year.
Methane (CH4)
Methane (swamp gas) is produced when plant material decays without oxygen. The largest sources of natural methane are swamps and marshes where fallen plants are decaying in the underwater mud.
Like carbon dioxide, methane absorbs infrared energy. In fact, one molecule of methane is 20 to 30 times better at absorbing infrared energy than a molecule of carbon dioxide.

In swamps, methane gas bubbles to the surface soon after it is produced. However, large pockets of methane gas can become trapped between layers of soil and rock. Gas trapped in this way is called natural gas. Huge pockets of natural gas are often found near deposits of oil. Initially, the natural gas was seen as a waste product. However, people found the gas could be piped to homes and industries and used as fuel. Some of the methane added to the atmosphere each year by human activities comes from leaky gas pipes.
However, most of the methane added to the atmosphere every year comes from agricultural activities. For example, the soil in which rice plants grow must be covered with water. The hollow stem of the rice plant serves as a pipe through which the methane produced by the decaying matter beneath the water escapes into the air. With many new rice paddies constructed to feed the world’s growing population, the amount of methane in the atmosphere is increasing rapidly.

Domesticated cattle, sheep, goats, and camels produce methane in their digestive systems. One cow produces approximately one-half pound of methane per day. There are estimated to be 3.3 billion domesticated animals in the world. Their population has increased as human societies have grown.
Microbes in the guts of termites also produce methane. One of the arguments against the practice of cutting rain forests is that the increase in termite activity that occurs when trees are cut may add significant quantities of methane to the atmosphere.
About 10% of all methane produced is the result of burning wood. Wood is still burned as a primary fuel in some parts of the world. The gas is also released during coal mining operations and landfills.
Can an Ice Cube Burn?
Yes, if it’s a cube of ice-like material taken from the tundra in the Arctic. When organic material decays in the frozen wastes of the Arctic, the methane can sometimes get trapped in little cage-like structures of ice crystals. The same phenomenon can happen in the sediments on the shores of the Arctic Sea. When the ice crystals melt, methane is released and can burn. One of the concerns of scientists is, if the tundra and the Arctic Seas warm, large quantities of the frozen methane could be released, significantly adding to the concentration of greenhouse gases in the atmosphere.
QUESTION 7.1. Would release of methane from Arctic Sea be an example of positive or negative feedback? Explain.
Nitrous Oxide (N2O)
Nitrous oxide is another greenhouse gas. It is produced naturally by microbes in the soil and from the burning of wood in forest fires. About one third of the nitrous oxide in the atmosphere is the result of human activities. The increase in concentration comes from the large-scale use of chemical fertilizers and the burning of fossil fuels for energy, primarily in automobiles.

The concentration of nitrous oxide is increasing at the rate of about 0.2% per year. Although its rate of increase is relatively small, it is a cause for concern because nitrous oxide is 250 times more efficient than carbon dioxide in absorbing infrared energy. In addition, nitrous oxide breaks down slowly. Once it is in the atmosphere, a molecule may have a lifetime of more than 150 years.
Recently, scientists at the University of California at San Diego identified an industrial source of nitrous oxide. They found the process of manufacturing an acid, from which nylon is made, gives off nitrous oxide. For every kilogram of nylon produced, a kilogram of nitrous oxide is generated and enters the atmosphere. That process alone accounts for one tenth of the increase of nitrous oxide released into the atmosphere each year.
Chlorofluorocarbons (CFCs)
Chlorofluorocarbons are gases that contain chlorine, fluorine, and carbon. In contrast to the other greenhouse gases that have always been present to some extent in the atmosphere, there is no natural source of CFCs. They are completely a product of the chemical industry, and were developed for use in refrigerators, air conditioners, and as a cleaning fluid in certain industries.

In January 1999, the concentration of CFC-11 was measured to be 260 parts per trillion (ppt), and CFC-12 was measured at 528 ppt. A few hundred parts per trillion may not seem like a lot, but CFCs have a fantastic capacity to trap infrared energy. Each CFC molecule can trap between 17,000 and 20,000 times the energy that is absorbed by a single molecule of carbon dioxide. In all, the family of CFC gases accounts for about 12% of the greenhouse warming observed in recent years.
CFCs were increasing in the atmosphere at a rate of about 7% per year until 1993, when an international agreement (the Montreal Protocol) called for industrial nations to stop using these gases. The primary reason for this treaty was that CFCs were known to destroy ozone in the stratosphere. But the treaty also had a beneficial effect helping reduce CFCs in the lower atmosphere, where it acted as a greenhouse gas. Due to the Montreal Protocol, new refrigerators and air conditioners must now use different gases. However, since it takes 50 to 100 years for CFC molecules to break down, the CFCs already in the atmosphere will remain with us for a very long time.
Ozone (O3)
A “normal” oxygen molecule in the atmosphere consists of two oxygen atoms linked together by chemical bonds. An ozone molecule is a temporary partnership of three oxygen atoms. Ozone is formed when an energy source such as ultraviolet radiation from the Sun, an electrical spark, or lightning breaks the bonds between oxygen atoms in a “normal” oxygen molecule, creating individual oxygen atoms. An individual oxygen atom (O) can combine with an oxygen molecule (O2) to produce a molecule of ozone gas with three atoms of oxygen (O3).
Ultraviolet light striking normal oxygen molecules in the upper atmosphere has resulted in the ozone layer, which is a slight concentration of ozone gas (about 12 parts per billion) about 30 to 40 kilometers above Earth’s surface. It does not consists of ozone alone; there is, however, a slight increase in the concentration of ozone at that level. People who have heard about the “hole in the ozone layer” often mistake this problem with the cause of global warming. Actually, ozone in the upper atmosphere has very little to do with global warming. So it’s important to describe the role that ozone plays in both the upper and lower atmosphere.
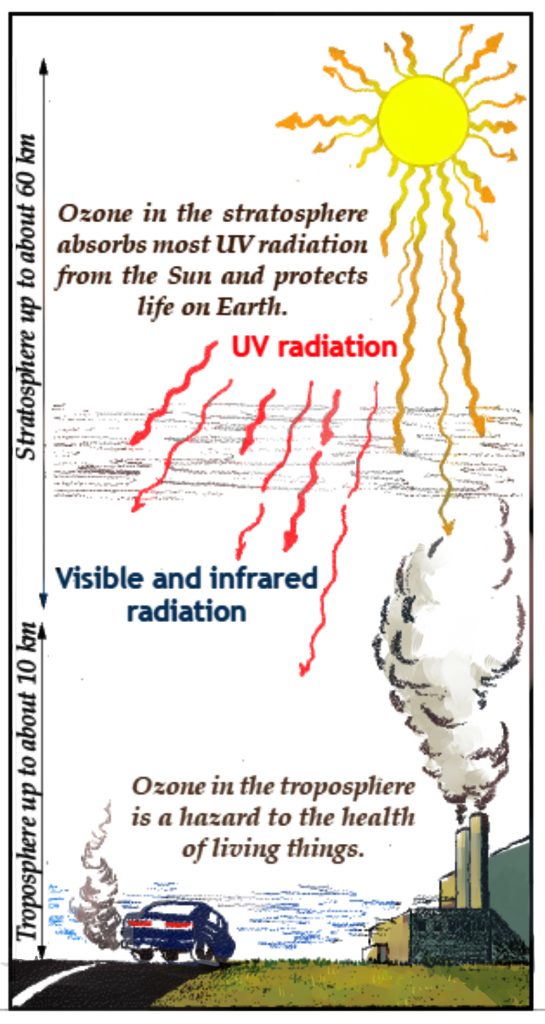
In the Stratosphere – High above the surface of Earth, ozone shields living things from the lethal effects of the Sun’s ultraviolet radiation. The ozone layer permits living organisms to live on land. Without this important gas, life would be restricted to the oceans.
In the 1980s it was discovered that CFC gases produced by the chemical industry have gradually drifted into the upper atmosphere where these gases have begun to destroy the ozone layer. Scientists are concerned that if the trend continues, people and animals will be exposed to more of the Sun’s ultraviolet radiation, and will be more likely to contract melanoma, a deadly form of skin cancer, and cataracts, a disease that clouds the cornea of the eye.
In the Troposphere – Since ozone absorbs infrared energy, it functions as a greenhouse gas when it is in the lower atmosphere, where it intercepts infrared energy from Earth’s surface. Ozone is one of the components of urban smog, and because of its poisonous chemical properties, it is considered a serious health hazard. The amount of ozone varies widely, but the average concentration of this gas is increasing in urban areas. Automobile exhaust accounts for about 75% of the ozone production in the troposphere.
We want ozone in the upper atmosphere to block harmful ultraviolet rays from the Sun, but we don’t want it near Earth’s surface, where it poisons living things and acts as an added greenhouse gas. Both ozone gas and CFCs act as greenhouse gases in the lower atmosphere.
II. Summary of the Gases That Contribute To The Increased Greenhouse Effect
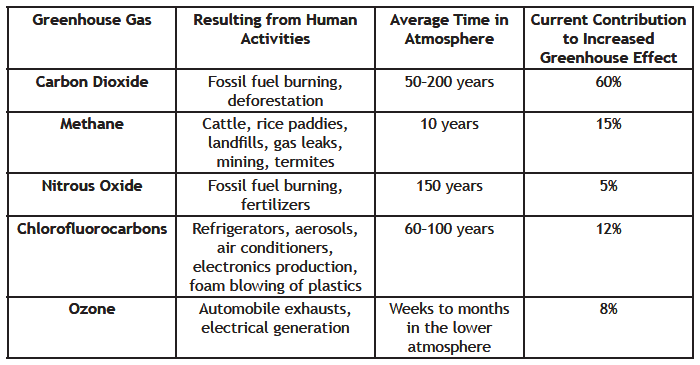
See also the Environmental Protection Agency’s web page, Overview of Greenhouse Gases, https://www.epa.gov/ghgemissions/overview-greenhouse-gases.
III. All Together Now!
Discussions about the problem of potential global climate change very often focus on the increase in atmospheric carbon dioxide. The burning of fossil fuels is so much a part of our lives that the production of carbon dioxide is easily evident. However, the other greenhouse gases are becoming increasingly important. As the chart above shows, the additional concentration of carbon dioxide in the air due to human activities accounts for about 60% of the increased greenhouse effect, while all the other greenhouse gases together account for about 40% of the effect. Together, these gases create the current concern about global warming because they are all increasing.
You may be wondering why water vapor is not included in the chart. As you know, water vapor is a potent greenhouse gas. For billions of years water vapor has helped keep the atmosphere warm enough for life. Although water vapor may be increasing as a result of a warming climate, it is not added to the atmosphere by human activities, and scientists do not know for sure what its effect on the climate will ultimately be. The chart includes only those gases that are contributing to the increased greenhouse effect.
IV. Why Is the Concentration of Greenhouse Gases Increasing?
The increase in greenhouse gases can be attributed to three major factors: population growth, industrialization, and deforestation.

Population Growth
At the start of the 20th century there were 1.6 billion people worldwide. Now there are 6 billion people on Earth. The world’s population is exploding. According to projections, there may be 8.5 billion by the year 2025. Each person added to the world’s population increases the human activities that are responsible for the growing levels of greenhouse gases.

Industrialization
While population is a major factor in creating an increase in the concentration of greenhouse gases, the main cause is industrialization—the growing number of automobiles, power plants, and factories that burn fossil fuels. Although the United States accounts for only 5% of the world’s population, because of its high level of industrialization, it produces more greenhouse gases than any other country in the world.
However, as developing countries become more and more industrialized, the burning of fossil fuels is increasing worldwide.

Deforestation
Forests are not only valuable as homes for the world’s wildlife; they also absorb vast quantities of carbon dioxide from the atmosphere. When trees grow, they absorb carbon dioxide from the air and store the carbon in wood and leaves. As forests are burned to create farmland and residential areas, the carbon stored in the trees reenters the atmosphere. While forests are increasing in some areas, such as the Northeastern United States, worldwide forest cover is rapidly decreasing.
According to the Worldwatch Institute, “Almost half the forests that once covered the Earth are gone, and deforestation is expanding and accelerating. The health and the quality of remaining forests are declining.”

